Our research focuses on understanding how signaling pathways impact immune function. We began our work on the anti-viral cytokine, type I interferon (IFN-a/b). While its antiviral properties were well characterized, very little was known about its effects on adaptive immune cells. We made a number of key discoveries that revealed how this cytokine shapes the T cell response, particularly in humans, and our interest in this area continues to this day. From our initial studies in T cells, we recently turned our attention to the interaction of immune cells with the nervous system. In particular, we are currently studying how the sympathetic nervous system regulates both innate and adaptive immune responses to viral infections, such as influenza.
Current Ongoing Projects
Please open the sections below to read about each project.
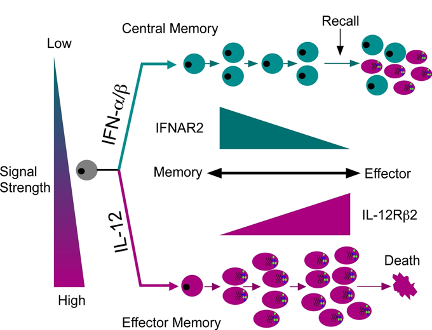
Reciprocal roles of IL-12 and IFN-a/b in shaping T cell fates
Our interest in interferon began with studying the IFN receptor and its downstream activation of the JAK/STAT pathway. We identified a unique molecular interaction between STAT2 and STAT4 that promoted STAT4 activation in human T cells. Based on this, it was assumed that IFN could promote typical Th1 development, much like IL-12, which shared STAT4 activation in common. However, we found that IL-12 and IFN were not redundant and rather drove separate pathways of effector vs central memory T cell development. Our ongoing studies seek to define the downstream transcriptional pathways that emanate from these two disparate signaling pathways with the goal of understanding how memory development is shaped by these cytokines at the single cell level.
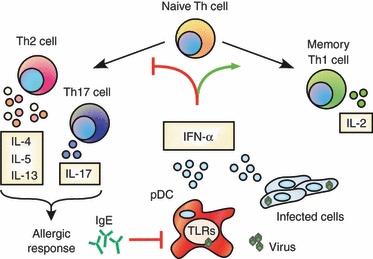
IFN-a/b blocks human Th2 development and cytokine secretion
CD4+ T cells are comprised of various subsets, which perform distinct functions of an immune response. One such subset, called "Th2" cells are involved in allergic reactions, such as allergic asthma. In studying the various effects of IFN on human T cells, we discovered that IFN potently blocked IL-4-mediated Th2 development in human CD4+ T cells. This was quite surprising as IFN does not have this effect on mouse cells. IFN suppressed Th2 development by inhibiting the expression of a key Th2-specific transcription factor called GATA3. In addition, we discovered that IFN could block the secretion of inflammatory Th2 cytokines such as IL-5 and IL-13, even from Th2 cells from allergic human subjects. Ongoing projects from these findings seek to understand the relationship between viral infections, which drive IFN production, and allergic diseases.
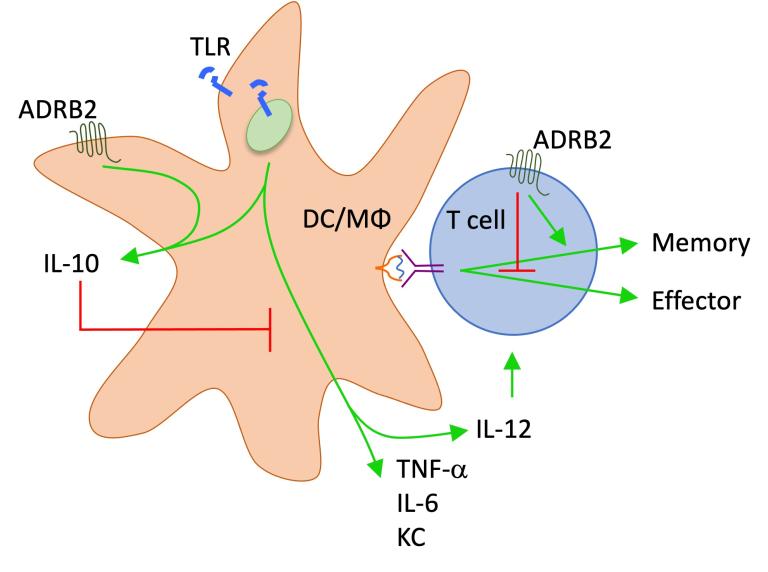
Epinephrine and norepinephrine suppress inflammation
Our early studies to characterize the transcriptional profile of human CD8+ T cells identified the adrenergic receptor expressed within effector and effector/memory subsets. At that time, very little was known about how that receptor or its ligands, epinephrine and norepinephrine, impacted the function of T cells. We found that activation of the b2-adrenergic receptor acutely suppressed cytokine secretion and lytic activity of both mouse and human effector CD8+ T cells, suggesting a direct connection between the immune and sympathetic nervous systems. This pathway suppressed innate cells as well by promoting IL-10 secretion from macrophages and dendritic cells in response to pathogens. These discoveries highlight the important role of neural signaling in limiting the extent of inflammation and damage resulting from infection.
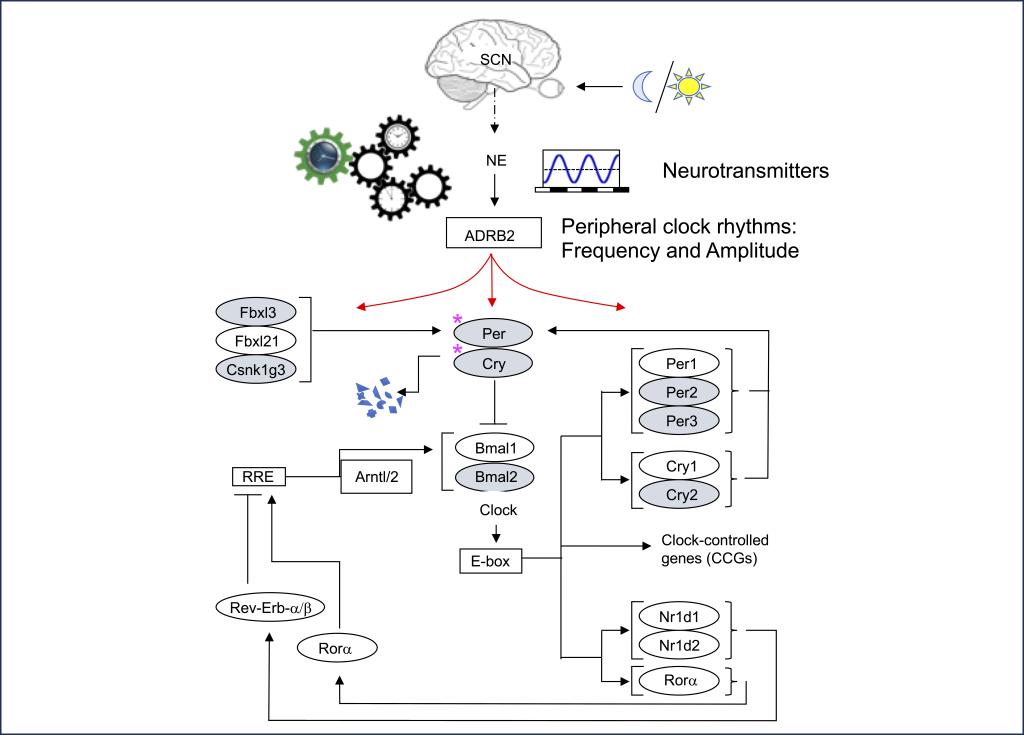
Neural regulation of circadian rhythms in immune cells
Virtually every organism on earth in some way synchronizes their biological functions to earth's 24 hour rotation. Light/dark cycles are perhaps the most well-characterized timers, or entrainers, of circadian rhythms. In higher organisms, light is converted to biological inputs that regulate the periodic expression of a set of core circadian genes. In turn, the oscillation of these genes regulate various downstream pathways that operate in a "time-of-day"-dependent manner, such as sleeping and eating. These downstream pathways also regulate the function of tissues and organs through sensing various neurotransmitters and hormones that oscillate through a 24 hr light/dark cycle. Indeed, immune cells display circadian rhythms, and immune responses to pathogens and vaccines can vary depending upon the time of day of exposure or responses to chronic inflammation. We are currently working to understand how various oscillating neurotransmitters regulate circadian rhythms in immune cells and how this impacts time-of-day-dependent immune responses to pathogens.