The Pan Laboratory has a long-standing interest in understanding the molecular mechanisms underlying growth control and tissue homeostasis. We tackle this question using a combination of Drosophila and mouse genetics, biochemistry, cell, and chemical biology approaches. Early work from our lab elucidated the molecular function of the Tsc1 and Tsc2 tumor suppressor genes, linking Tsc1/Tsc2 to Rheb and TOR signaling. This work provided the key molecular insight for the current use of mTOR inhibitors in the treatment of Tuberous Sclerosis. Most significantly, we have discovered and characterized a novel signal transduction pathway, the Hippo pathway, which controls organ size in all animals. Starting with Drosophila as a model, we systematically decoded, in a stepwise manner, the key molecular components of the Hippo pathway, including its core kinase cascade, the downstream transcriptional machinery, and multiple upstream regulators. Extending beyond Drosophila, we demonstrated a conserved role for Hippo signaling in mammalian tissue growth, regeneration, and tumorigenesis, and elucidated the molecular mechanism by which the classic tumor suppressor and human disease gene NF2/Merlin controls tissue growth. Our current efforts are aimed at further elucidation of the mechanisms, regulation, and evolution of Hippo signaling, as well as the discovery of new growth control pathways.
A unique strength of the Pan Lab is the combination of multiple experimental models (Drosophila, mice, and cell culture) and approaches (genetics, biochemistry, and cell/chemical biology). In doing so, we leverage the unique strengths of each experimental model/approach to uncover universal principles of growth control that are conserved across large evolutionary distances. Such synergy also provides a well-rounded training environment for graduate students and postdocs. Many previous trainees from the Pan Lab have gone on to successful careers in academia, industry, and government.
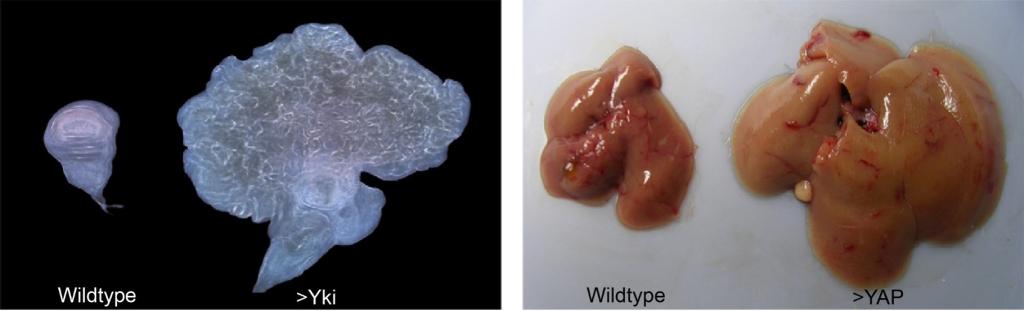
The Hippo pathway as a conserved size-control mechanism in flies and mammals
(Left) Wing imaginal discs from a wild-type fruit fly and a transgenic fly in which the Hippo pathway effector Yki is overexpressed. Yki overexpression leads to massive tissue overgrowth.
(Right) A normal mouse liver and a transgenic mouse liver overexpressing YAP, the mammalian counterpart of Yki. Like its Drosophila counterpart, overexpression of YAP leads to a massive increase in organ size.
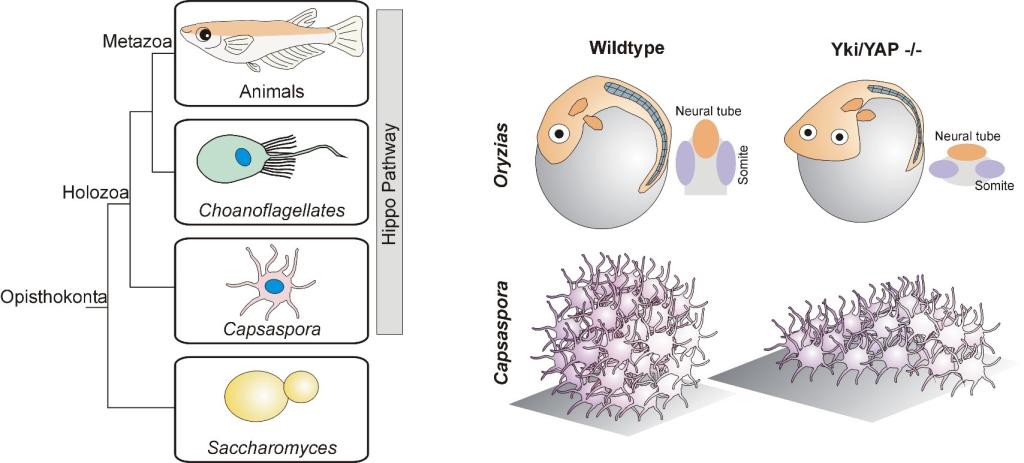
An ancestral and conserved role for the Hippo pathway in multicellular morphogenesis
(Left) Phylogenetic tree showing conservation of the Hippo pathway in close unicellular relatives of animals.
(Right) Loss of Yki/YAP leads to flattened 3D shape in both medaka fish embryos and multicellular aggregates of the unicellular organism Capsaspora.