Energy regulation is a highly integrated process that relies on careful coordination of energy availability and expenditure. This becomes especially critical during periods like infection, when the immune response increases demand and sickness behaviors like anorexia reduce supply. Our work involves understanding how the brain regulates energetics, i.e. metabolism and immune function, during infection. Our projects broadly fall into two categories:
Understanding leptin’s role in infection: Leptin is an adipokine that is released from adipose tissue and signals to the hypothalamus to affect food intake, metabolism, and energy expenditure. Our recently published work (Andres-Rojas, et al, JCI 2024) demonstrates that leptin might mediate sympathetic signaling that preserves heart rate and core temperature during infection. We are trying to identify the mechanism behind this phenomenon.
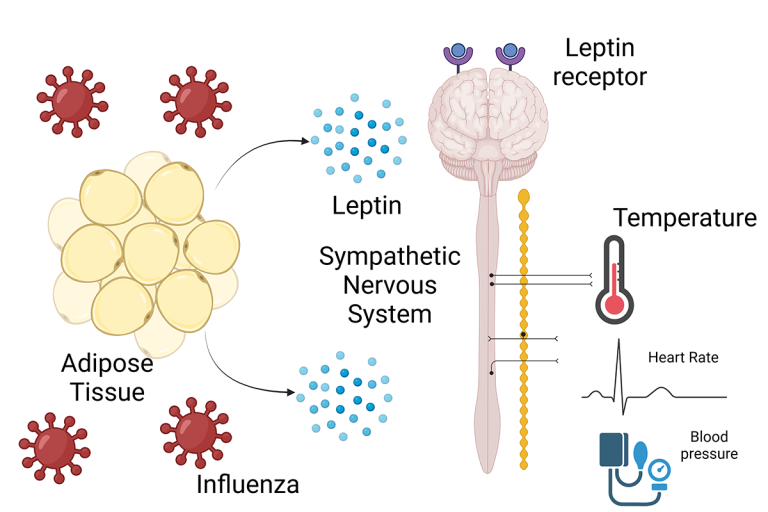
Understanding adipose tissue metabolism during infection: In collaboration with the Patel Lab at UTSW, we are investigating the role of lipolysis and the downstream metabolism of fatty acids in infection.