We study how cells shift from healthy function to early disease by directly visualizing molecular and nanoscale changes inside cells in their native environment.
Using cryo-electron tomography of intact neurons and disease-relevant cell models, together with quantitative biophysics and multi-modal imaging, we examine how normally functional proteins—such as alpha-synuclein—misassemble, alter cellular compartments, and destabilize the systems that maintain neuronal health.
To complement these in-cell studies, we also use cryo-electron microscopy to determine higher-resolution structures of disease-associated components extracted from cells, including amyloid fibrils, both alone and in complex with molecular binders selected based on binding affinity.
Our earlier work defined the structure and molecular composition of Lewy bodies and Lewy neurites in human brain tissue. Building on this foundation, we now focus on earlier stages of disease, connecting molecular organization to organelle dysfunction in order to identify the points at which cellular failure first emerges and may still be prevented.
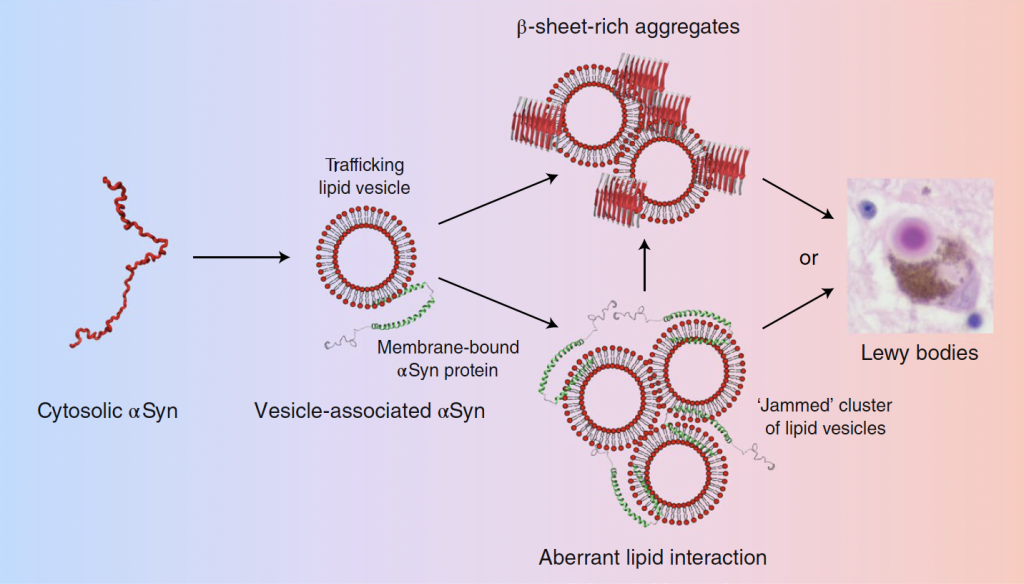
 Shahmoradian, Sarah H., et al. "Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes." Nature Neuroscience 22.7 (2019): 1099-1109.
Shahmoradian, Sarah H., et al. "Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes." Nature Neuroscience 22.7 (2019): 1099-1109. 
Our lab at UT Southwestern visualizes and molecular secrets and clues directly inside cells—capturing how disease-associated proteins interact with membranes, remodel organelles, and disrupt specific neuronal sub-compartments such as synapses and axons in aging and disease, including Parkinson’s and Alzheimer’s disease.
Many of our fundamental structural biology studies are performed in close collaboration with the cell biology lab of David Sanders. The Sanders Lab’s genetically precise cell models of RNA and protein aggregation integrate with the Shahmoradian Lab’s cryo-electron tomography, cryo-electron microscopy, and cryo-correlative light and electron microscopy (cryo-CLEM) to resolve pathogenic processes in their native cellular context. In parallel, we develop new experimental interfaces and technologies to push the limits of what can be visualized at the nanoscale, with the goal of identifying early structural events that precede irreversible cellular failure.
Scientific Philosophy
Our research is broad by necessity. Across the Shahmoradian and Sanders labs, we share a joint scientific philosophy rooted in curiosity-driven inquiry, rigorous hypothesis testing, and choosing methods based on the question rather than precedent or trend. At times this leads to careful, incremental progress. At other times, stepping outside established models opens space for genuinely new insight. When that happens, we are willing to let go of the original question and follow where the biology leads.
This approach carries uncertainty, but it is also where discovery lives. Scientific progress is rarely linear, and productive detours often provide the clearest molecular clues about how cells and systems truly function. When obstacles arise, we rely on open communication and collaboration across groups. Transparency strengthens science; it does not weaken it.
The Shahmoranders, pictured above, reflect this shared culture at the intersection of structural biology, cell biology, and neurodegeneration. We are recruiting at multiple levels. If you are interested in asking hard questions and building careful, creative science, please reach out to David and/or Sarah to discuss opportunities for collaboration or training.