The Sorrell Laboratory utilizes integrative approaches that include metabolomics, transcriptomics, organoid cultures, live microcopy, and animal models, to investigate fundamental pathways that control the uptake of nutrients and the biosynthesis of macromolecules in proliferative cells. Our long-term goal is to develop strategies to interfere with nutrient uptake and utilization in cancer cells by applying dietary and pharmacologic interventions. Our main areas of research are:
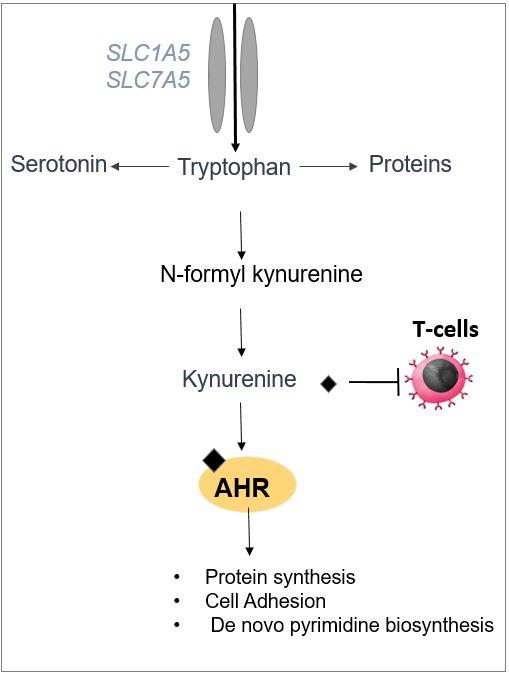
Exploring increased tryptophan uptake and metabolism as a vulnerability of cancer cells
We discovered that the growth-promoting transcription factor MYC drives the expression of genes that facilitate the uptake and metabolism of tryptophan to generate an active catabolite named kynurenine, thus eliciting a dependency on kynurenine for proliferation. We found that kynurenine activates the transcription factor aryl hydrocarbon receptor (AHR) in cancer cells (Genes & Development, 2019) and that AHR drives the transcription of genes involved in ribosome biogenesis (Genes & Development, 2018) and nucleotide biosynthesis (JBC, 2020) which are involved in the nucleolar activity (PLOS Genetics, 2020). Currently, we are developing new strategies to limit tryptophan uptake and metabolism and determining their ability to limit cancer cell growth in vivo.
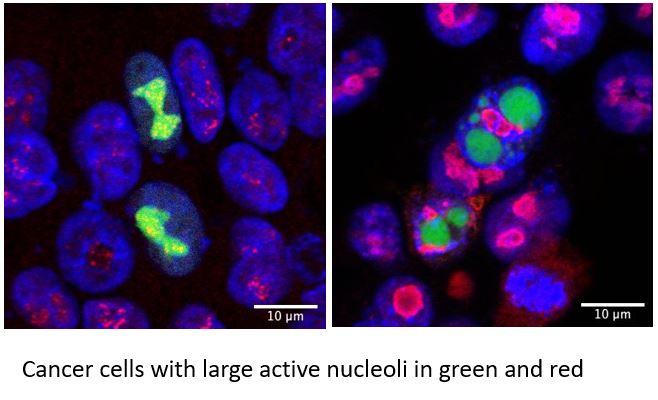
Dissecting novel regulators of nucleolar activity in rapidly proliferating cells
Our discoveries on cell growth and protein synthesis motivated our interest in the fundamental mechanisms regulating nucleolar assembly and activity to match the specific biomass requirements of proliferative cells. Using a combinatorial approach to discover uncharacterized genes necessary for nucleolar activity, we identified novel and critical regulators of nucleolar morphology, and ribosome biogenesis. We are currently studying how these factors organize the architecture of the nucleolus establishing functional hubs that facilitate ribosome biogenesis specifically in cancer cells.