Project 1: The ILLUMINATE Study
Our clinical research team is conducting a Phase-2 clinical trial with a drug, pegsitacianine based on the fluorescent nanoparticles developed in our lab. This study is to investigate whether pegsitacianine can be used to image head and neck cancers as well as unknown primary cancers (UPC) of the head and neck after being given intravenously. More precise detection of unknown primary cancers and precise delineation of cancer margins can potentially assist surgeons intraoperatively and allow more complete tumor resections. The study is divided into 2 parts:
Part 1
Patients with visible head and neck cancer will be given pegsitacianine before undergoing oncologic surgery and imaged to detect cancer margins.
Part 2
Patients with unknown primary cancer (UPC) of the head and neck will be given pegsitacianine before undergoing exam under anesthesia or laryngoscopy intended to identify their primary cancer.
For more details refer to the attached flyer and ClinicalTrials.gov, Identifier: NCT05576974. If you are interested in participating in the trial, please contact the Department of Otolaryngology by phone at research department at Email.
Reference:
Selected publications:
- Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, Zhao T, Sumer BD, DeBerardinis RJ, Gao J. A Nanoparticle-based Strategy for the Imaging of a Broad Range of Tumours by Nonlinear Amplification of Microenvironment Signals. Nat. Mater. 2014, 13, 204-212. PDF
- Zhao T, Huang G,Yang S, Ramezani, S, Li Y, Wang Y, Ma X, Xie XJ, Thibodeaux J, Sun X, Sumer BD, Gao J. A Transistor-like pH Nanoprobe for Tumour Detection and Image-Guided Surgery. Nature Biomed. Eng. 2016, 1, 0006. (Featured by Science daily news, Dallas Morning News, NBC5, and CBS11) PDF
- Huang G, Zhao T, Wang C, Nham K, Xiong Y, Gao X, Wang Y, Hao G, Ge WP, Sun XK, Sumer BD, Gao J. PET Imaging of Occult Tumours by Temporal Integration of Tumour-Acidosis Signals. Nature Biomed. Eng. 2020, 4, 314. PDF
- Bennett ZT, Feng Q, Bishop JA, Huang G, Sumer BD, Gao J. Detection of Lymph Node Metastases by Ultra-pH-Sensitive Polymeric Nanoparticles. Theranostics. 2020, 10(7), 3340-3350. PDF
- Voskuil FJ, Steinkamp PJ, Zhao T, van der Begt B, Koller M, Doff JJ, Jayalakshmi Y, Hartung JP, Gao J, Sumer BD, Witjes MJH, van Dam GM. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nature Comm. 2020, 11, 3257. PDF
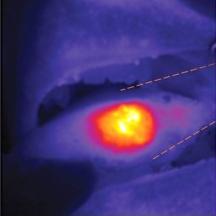
Using a fluorescent camera during surgery, nanoparticles illuminate the tumor in this patient's tongue guiding the surgeon during the procedure.
Project 2: Tumor Acidosis: Hallmark of Cancer
Tumor Acidosis: Hallmark of Cancer
Cancer cells are metabolically active and use the glycolytic pathway to generate energy, resulting in high uptake of glucose and preferential production of lactate (Warburg effect). Exportation of lactic acid/CO2 leads to a pH drop within the tumor microenvironment. Our lab has developed a series of pH-activatable fluorescence probes with different pH transitions attached to different fluorescent dyes, such as rhodamine, Cy5 or ICG. These pH-activatable fluorescence probes give us the ability to image tumors, study tumor acidosis (Warburg effect), and investigate the tumor immune microenvironment (TIME). To investigate potential correlations between TIME and tumor acidosis, our lab used 10X Genomics' Xenium Prime spatial transcriptomics. This has allowed us to analyze differential gene expression in terms of cell type and location at single-cell resolution, as well as comparing HPV+ with HPV- tumors, and unknown primary tumors with their associated lymph node metastasis. Through a novel integration method, our lab has added each sample's associated pH-activatable fluorescence probe data at the single-cell level, providing further avenues for downstream analysis.
Research Interest
Surgical specimens collected from patients enrolled in our tissue biobank are used to study the tumor immune microenvironment (TIME) including the effects of acidosis on infiltrating immune cells. This is especially relevant to understanding the differences between HPV+ and HPV- squamous cell carcinoma since oropharyngeal cancers arise in tonsillar tissue that is immunologically active.
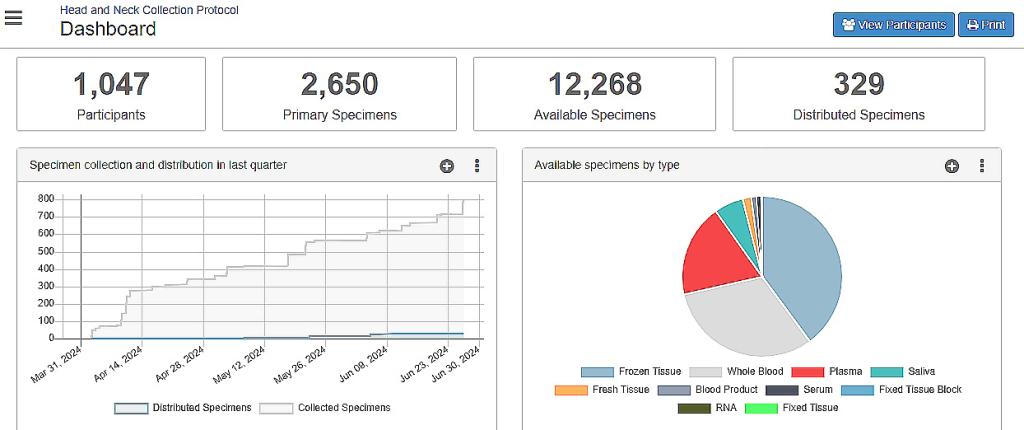
Our Laboratory Tissue Biobank
Our biological specimen repository has 10,000+ specimens from 900+ patients that are preserved using a variety of advanced techniques intended to allow long-term access to live cells, RNA, DNA and proteins from solid and liquid specimens. At the forefront of biomedical research, our repository serves as a pivotal resource for the collection, preservation, and distribution of high-quality specimens sourced from patients with head and neck malignancies and benign tumors. With a commitment to accelerating scientific discoveries, our repository plays a crucial role in facilitating interdisciplinary investigations, fostering collaborations, and ultimately paving the way for innovative therapies and personalized treatment strategies. All studies are compliant with the policies of the UT Southwestern Human Research Protection Program (HRPP) Department, are HIPAA compliant and IRB approved (STU 092013-032).
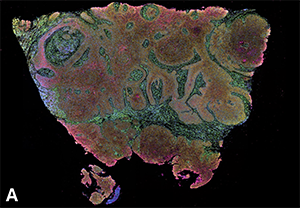
Head and neck squamous cell carcinoma surgically removed from a patient intravenously infused with ONM-100 (pegsitacinaine) nanoparticles for fluorescence-guided surgery. (A) Cell type clustering based on Xenium spatial transcriptomics, analyzing the expression of ~5000 genes via mRNA profiling.
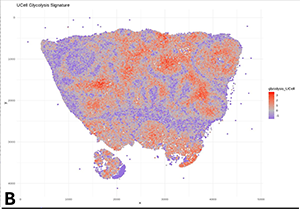
(B) Representative transcriptomic data highlighting glycolysis-related pathways.
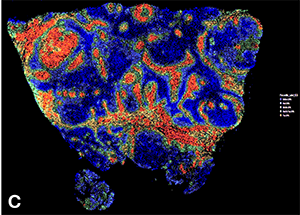
(C) Quantification of pegsitacinaine fluorescence intensity across distinct tumor regions. Xenium analysis of fluorescence data provides insights into cancer-stroma interactions and nanoparticle activation in vivo"
Project 3: Our Laboratory Tissue Biobank
Our biological specimen repository has 10,000+ specimens from 900+ patients that are preserved using a variety of advanced techniques intended to allow long-term access to live cells, RNA, DNA and proteins from solid and liquid specimens. At the forefront of biomedical research, our repository serves as a pivotal resource for the collection, preservation, and distribution of high-quality specimens sourced from patients with head and neck malignancies and benign tumors. With a commitment to accelerating scientific discoveries, our repository plays a crucial role in facilitating interdisciplinary investigations, fostering collaborations, and ultimately paving the way for innovative therapies and personalized treatment strategies. All studies are compliant with the policies of the UT Southwestern Human Research Protection Program (HRPP) Department, are HIPAA compliant and IRB approved (STU 092013-032).
Last updated 9/10/2024
See the OpenSpecimen Dashboard
(Available only through the UTSW network or VPN)