As chemists at a medical center, we are integrating newly developed chemical methods into medicinal chemistry partnerships with the drug development community at UT Southwestern. For example, in collaboration with Professors Bruick, Gardner, and MacMillan, we have developed an enantioselective cycloaddition for the synthesis of an isoform-selective inhibitor (Kd=28 nM) of hypoxia inducible factor 2α (HIF-2α), a transcription factor that is a key component in the deregulated growth and remodeled metabolism of cancer cells. Although transcription factors are commonly considered ‘undruggable’ biological targets, we have generated ligands that inhibit the complexation of HIF-2α with its essential protein partner.
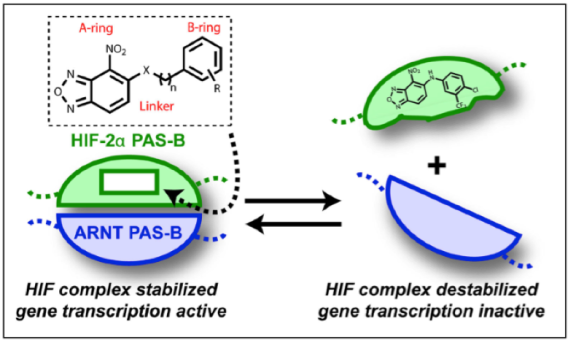
The most active inhibitors developed in this collaboration were licensed to Peloton Therapeutics, a local startup pharmaceutical company that developed a clinical candidate. Peleton was acquired by Merck in 2019, and in 2021 the FDA approved belzutifan (Welireg) for patients with VHL disease who require therapy for renal cell carcinoma, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors.
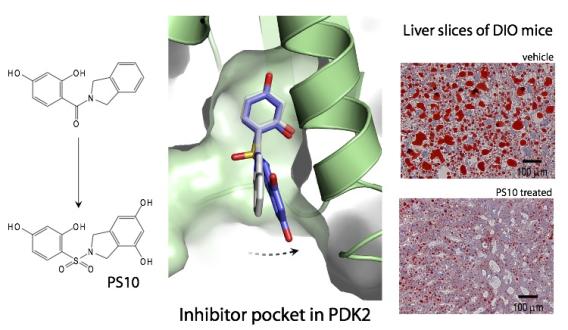
In collaboration with Professor Chuang, we have also utilized structure-based design to access an isoform-selective chiral inhibitor (Kd=250 nM) of pyruvate dehydrogenase kinase 2, which is a pharmacological target for obesity and type 2 diabetes, and a selective inhibitor of the branched-chain α-ketoacid dehydrogenase kinase, a pharmacological target for maple syrup urine disease. As a result, the Tambar and Chuang Groups jointly filed a patent for the generation and use of inhibitors of mitochondrial pyruvate dehydrogenase kinase isoforms 1-4.
Ongoing projects include development of inhibitors for various enzymes and protein-protein interactions involved in cell growth regulation, bacterial processes, and other clinical indications in collaboration with laboratories at UT Southwestern.