Ultrasound-Guided Drug Delivery
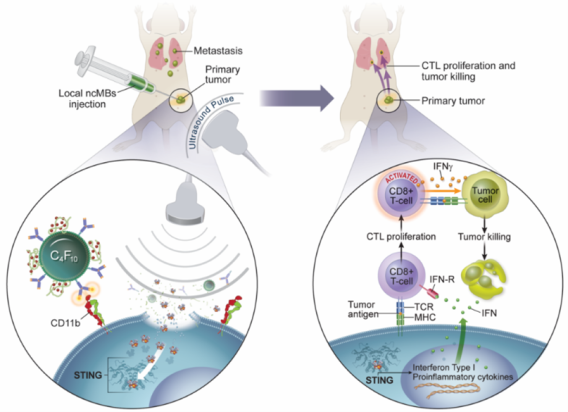
There is an urgent and unmet need to develop more effective immunotherapy strategies for cancer patients. In order to generate optimal antitumor immunity, both the innate and adaptive branches of the body’s immune systems need to be engaged. This understanding has led to the development of new immunotherapies that target regulators of innate immune systems, including agonists that stimulate the cytosolic DNA sensor, cyclic GMP-AMP Synthase-Stimulator of Interferon Genes pathway (cGAS-STING) pathway. However, STING agonists such as cyclic GMP-AMP (cGAMP) cannot penetrate the cell membrane easily, which is required to activate downstream antitumor immune responses, and thus severely limits their clinical potential. We have developed a microbubble-assisted ultrasound (US)-guided immunotherapy for cancer (MUSIC) that reengineers clinically approved microbubbles to load and deliver
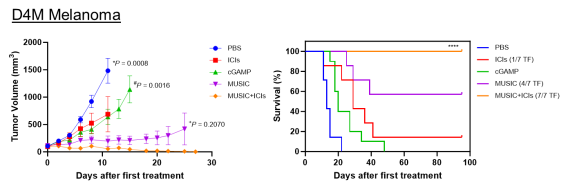
We have developed a microbubble-assisted ultrasound (US)-guided immunotherapy for cancer (MUSIC) that reengineers clinically approved microbubbles to load and deliver cGAMP into APCs under US stimulation with spatiotemporal control with the use of a preclinical sonoporator. We showed that MUSIC produced robust STING activation and type I interferon responses in APCs and more efficiently primed antigen-specific CD4+ and CD8+ T cells in vitro. In collaboration with Wen Jiang, MD, PhD demonstrated that these immune stimulatory effects directly translated into antitumor effects against syngeneic orthotopic (EO771) and metastatic (4T1) murine breast cancer models. Both models showed dramatic anti-tumor responses following local treatment of the primary tumor, with 60% of animals becoming tumor-free after 50 days in the case of the EO771 tumors and a significant decrease of the systemic disease burden including lung metastases in the case of the 4T1 breast cancer.
Li, X., Khorsandi, S., Wang, Y. et al. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat. Nanotechnol. 17, 891–899 (2022). https://doi.org/10.1038/s41565-022-01134-z
Khorsandi S, Huntoon K, Lux J. Putting the sting back in STING therapy: novel delivery vehicles for improved STING activation. Front. Chem. Biol. 3 (2024). https://doi.org/10.3389/fchbi.2024.1386220
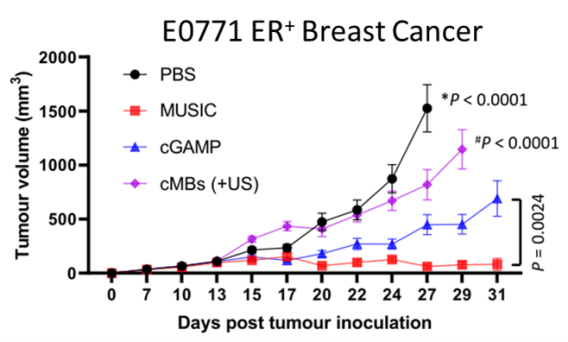
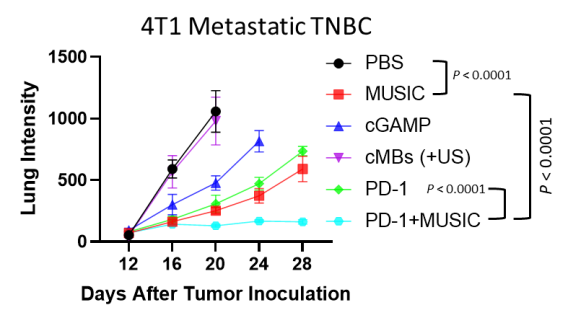
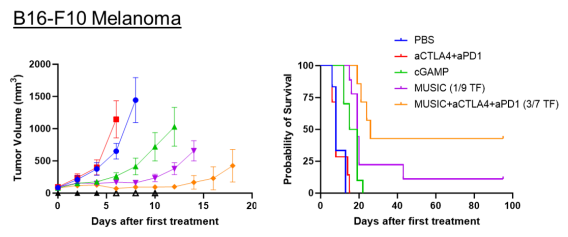
We also demonstrated the efficacy of MUSIC in syngeneic orthotopic melanoma (B16-F10, D4M.3A) models. While all D4M.3A tumor-bearing mice achieved complete remission with this approach in combination with inhibition checkpoint inhibitors, only 3 out of 7 B16-F10 tumor-bearing mice responded similarly. To address this limitation, we are now developing a complementary microbubble platform that delivers tumor-associated peptide antigens to APCs, enhancing antigen cross-presentation.
S. Khorsandi, K. Huntoon, Y. Wang, A. Woodward, A. Antony, C. Endsley, N. Hafeez, J. L. Edwards, N. McCuen, P. G. Alluri, B. Y. Kim, W. Jiang, J. Lux, Targeted STING Activation Using Modified Ultrasound-Responsive Microbubbles Enhances Immune Checkpoint Blockade Against Melanoma. Adv. Sci. 2025, 2416596. https://doi.org/10.1002/advs.202416596
Phase-Shift Ultrasound Contrast Agents
Acoustic droplet vaporization (ADV) of superheated perfluorocarbon nanodroplets (NDs) holds significant promise as an extravascular ultrasound contrast agent, with potential to advance ultrasound-guided therapeutic applications such as targeted drug delivery. Our research focuses on the design and formulation of NDs by exploring composition–structure–property relationships, aiming to expand diagnostic and therapeutic capabilities beyond the current limitations of microbubble-based technologies. We are actively pursuing several exciting projects in this area and welcome inquiries from those interested in joining our team or exploring collaborative opportunities.
Direct emulsification of stable superheated perfluorobutane nanodroplets by sonication: addressing the limitations of the microbubble condensation technique, Ultrasound in Medicine and Biology, 2024, 50, 445-452. https://doi.org/10.1016/j.ultrasmedbio.2023.12.008
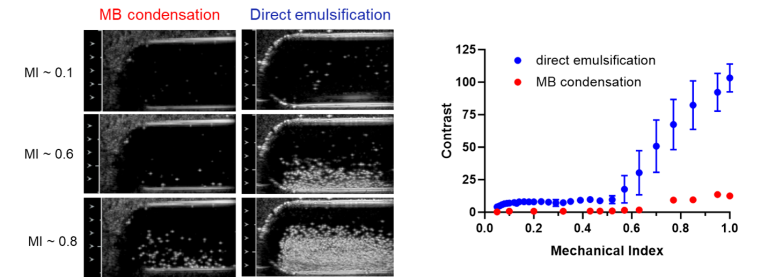
The dominant conclusion of this report is that nearly 100% of particles produced by direct emulsification of perfluorobutane (PFB) liquid at low temperature are PFB-filled, whereas the emulsion produced by microbubble (MB) condensation if the MBs are not washed, contains 2000 times more non-PFB filled than PFB-filled particles.
In Vivo Ultrasound Imaging of Macrophages Using Acoustic Vaporization of Internalized Superheated Nanodroplets, ACS Applied Materials and Interfaces, 2023, 15, 42413-42423. https://pubs.acs.org/doi/10.1021/acsami.3c11976

Positively charged nanodroplets containing low boiling point perfluorocarbon are internalized by macrophages and vaporize after being exposed to ultrasound pulses using a clinical scanner. Vaporization into microbubbles occurs without affecting cell survival or phagocytic function and is successfully achieved for at least 8 h after internalization both in vitro and in vivo.
Bubble Inflation Using Phase-Change Perfluorocarbon Nanodroplets as a Strategy for Enhanced Ultrasound Imaging and Therapy, Langmuir, 2020, 36, 2954-2965. https://pubs.acs.org/doi/10.1021/acs.langmuir.9b03647
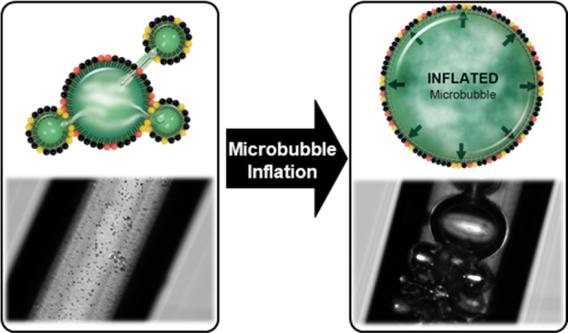

A new theory and method to inflate PFC gas bubbles have been developed and validated. This work demonstrates that the coexistence of liquid PFB NDs and gaseous PFB MBs leads to the dramatic inflation of the gas bodies, increasing their diameter by at least 2 orders of magnitude.
Fluorous-Phase Iron Oxide Nanoparticles as Enhancers of Acoustic Droplet Vaporization of Perfluorocarbons with Supra-Physiologic Boiling Point, J Control Release, 2019, 302, 54-62 https://www.sciencedirect.com/science/article/pii/S0168365919301592
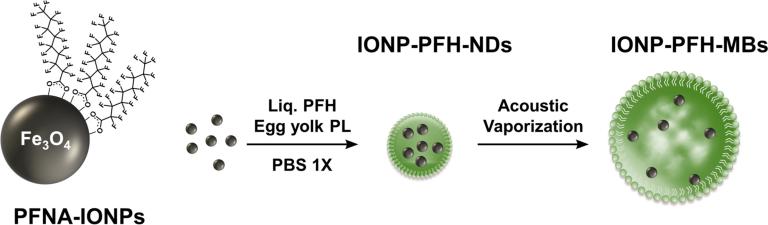
Using Perfluorohexane nanodroplets (PFH-NDs) loaded with 5% w/v iron oxide nanoparticles (IONPs), we were able to trigger acoustic droplet vaporization (ADV) at physiological temperature and at 42 °C using pressures that are just above the FDA-approved limit for clinical ultrasound scanners. ADV threshold for the 10 and 15% IONP emulsions at 42 °C were lower than the FDA limit. When IONP-PFH-NDs containing 5% IONP that passively accumulated in tumors by 5 h after IV administration were exposed to an ADV pulse in vivo, they converted to MBs detectable by ultrasound. Our findings are in agreement with the known concept that particles within a liquid provides nucleation sites that lower the ultrasound cavitation threshold
Novel Method for Formation of Monodisperse Superheated Perfluorocarbon Nanodroplets as Activatable Ultrasound Contrast Agents, RSC Advances, 2017, 7, 48561-48568. https://pubs.rsc.org/en/content/articlelanding/2017/ra/c7ra08971f

This work confirms that direct emulsification of low boiling point perfluorocarbon (such as perfluorobutane or “decafluorobutane” (DFB) and octafluoropropane (OFP)) into liquid nanodroplets for phase-shift ultrasound controlled vaporization is possible. Emulsions of DFB were stable for >18 days (entire observation periods) at 4 °C and >1 day at room temperature allowing further processing for functionalization and purification. More important, DFB formulations were stable for at least 2 h at physiologic temperature without spontaneous vaporization, allowing ample time for targeting and tissue accumulation. They transitioned into MBs in vitro only when exposed to ultrasound at low Peak Negative Pressure (PNP = 0.38 for OFP and 1.07 for DFB) producing marked enhancement on B-mode US imaging.
New Tools for Quantitative Ultrasound Imaging
Tissue-Mimicking Phantom for Liver Fat Quantification
We have developed an innovative methodology for creating ultrasound phantoms that replicate the fat vesicle size characteristic of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Our approach utilizes agar-based phantoms embedding stable peanut oil droplets as analogs for lipid vacuoles, effectively simulating fat accumulation in steatotic hepatocytes. These phantoms offer significant potential for validating quantitative imaging methods, particularly ultrasound-based diagnostic tools. Ultimately, our goal is to improve screening, diagnosis, patient management, and treatment response for individuals with metabolic syndrome and MASLD.
Lipid Microparticle-Based Phantoms Modeling Hepatic Steatosis for the Validation of Quantitative Imaging Techniques, Small Methods, 2025. https://doi.org/10.1002/smtd.202500043
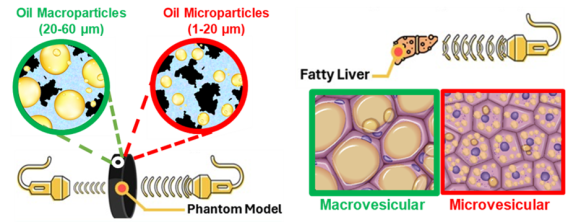 Example of phantom imaging
Example of phantom imaging Detection of Acute Thrombosis with New Activatable Ultrasound Contrast Agents
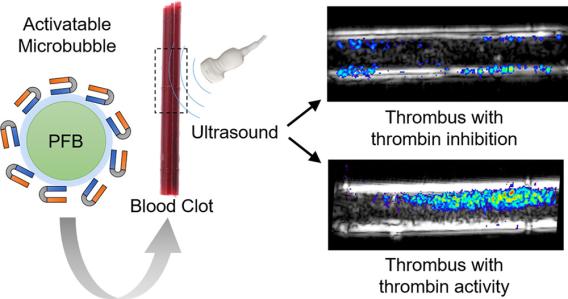
Patients with acute deep vein thrombosis (DVT) often present similarly to those with chronic DVT, making it challenging to distinguish between the two using traditional imaging techniques. This distinction is critical, as acute DVT requires immediate anticoagulation therapy to prevent potentially fatal pulmonary embolisms. To address this diagnostic challenge, we developed a thrombin-specific microbubble (MB) ultrasound contrast agent designed to identify patients who would benefit from anticoagulation therapy. The thrombin-specific activation of this contrast agent was successfully demonstrated using an in vitro flow phantom model.
Thrombin-Activatable Microbubbles as Potential Ultrasound Contrast Agents for the Detection of Acute Thrombosis. Jacques Lux, Alexander M. Vezeridis, Kenneth Hoyt, Stephen R. Adams, Amanda M. Armstrong, Shashank R. Sirsi, and Robert F. Mattrey. ACS Applied Materials & Interfaces 2017 9 (43), 37587-37596 DOI: 10.1021/acsami.7b10592
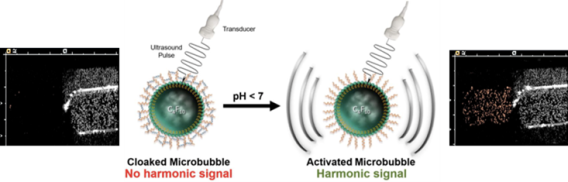
Building on our thrombin responsive platform, we reported the first example of hydrogel-cloaked microbubbles (MBs) and introduced activatable MBs that can be engineered to respond to a wide range of disease biomarkers. In a proof-of-concept study, we used pH as a model biomarker to demonstrate the versatility of this approach, which we aim to further develop for targeted detection of acute DVT using thrombin-sensitive MBs.
Microbubbles Cloaked with Hydrogels as Activatable Ultrasound Contrast Agents. Mary W. N. Burns, Robert F. Mattrey, and Jacques Lux. ACS Applied Materials & Interfaces 2020 12 (47), 52298-52306. DOI: 10.1021/acsami.0c12043
We are continuing to study the effects of pH on various lipid-based MB formulations. Specifically, we observed increased harmonic oscillation of phosphatidylcholine microbubbles (PC-MBs) in response to lower ambient pH using a clinical ultrasound scanner. While relative signal changes are interpreted clinically as mostly related to blood flow, pH effects could be significant contributors, particularly when imaging tumors. While our observation can be used clinically, it requires further research to isolate the effect of pH from other variables.
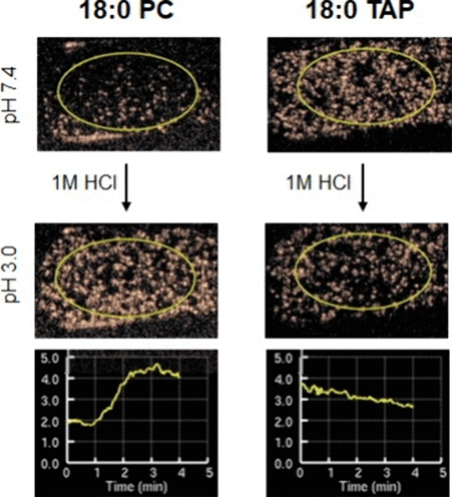

Modulating Nonlinear Acoustic Response of Phospholipid-Coated Microbubbles with pH for Ultrasound Imaging. Shariq Ali, Caroline de Gracia Lux, Katherine Brown, Connor Endsley, Adam Woodward, Robert Mattrey, and Jacques Lux. ACS Sensors 2024 9 (5), 2356-2363. DOI: 10.1021/acssensors.3c02382