The long-term goals of the Volk Lab are to elucidate the molecular and synaptic mechanisms of memory persistence, and to ultimately understand how changes in synapse function sculpt circuit-level information flow to facilitate the brain’s competing requirements for dynamic learning and persistent memory.
We combine animal-behavior studies with molecular biology, electrophysiology, and opto- or chemo-genetic methods in rodents to manipulate activity in discrete neuronal populations to investigate:
- How memory encoding and use changes across development under normal and pathological conditions.
- How sleep facilitates memory persistence.
We focus our studies primarily on the hippocampus, a brain region critical for learning and memory consolidation.
Memory Consolidation and Sleep
Sleep is a fundamental, conserved biological process, yet the essential physiological function of sleep remains an open question. Accumulating evidence supports a role for sleep in memory consolidation and maintenance of cognitive function. However, the neural substrates of and mechanisms underlying sleep-facilitated memory consolidation remain poorly understood.
One prominent hypothesis suggests that during sleep, ensemble patterns of neuronal activity which occurred during awake experience are reactivated to induce or consolidate synaptic plasticity, thus consolidating experience-relevant synaptic changes into long-lasting plasticity and memory.
An alternative hypothesis posits that sleep is a critical homeostatic process. This synaptic homeostasis hypothesis is based on observations that synaptic strength generally increases during waking periods and decreases during sleep. This ebb and flow suggests that progressive increases in synaptic strength accumulate in response to awake experience, and that downscaling of synaptic strength during sleep is a restorative process necessary to maintain neuronal firing rates at a level conducive to continued information processing.
While these two hypotheses are apparently contradictory, they are not mutually exclusive: it is possible that sleep facilitates a more complicated information triage in which neuronal representations of salient experience are strengthened amidst more global homeostatic downscaling of less important information.
We are using genetic tools to label and manipulate neuronal populations that participate in reactivation during sleep in order to investigate the synaptic changes induced by selective neuronal reactivation versus global downscaling in sleep, and to determine which of these changes are necessary for memory consolidation and maintenance of cognition.
 Is this why we sleep?
Is this why we sleep? Synaptic Mechanisms of Learning and Memory
The ability to learn, form lasting memories, and use stored information to guide future behavior are fundamental and conserved neurobiological processes.
However, the nature and efficacy of these processes are not static across the lifespan of an organism. Many cognitive functions, including learning and memory, mature during adolescence and also show age-related decline.
The importance of successful age-related transitions in neuronal function is highlighted by psychiatric and neurological disorders, including schizophrenia, bipolar disorder, and Alzheimer’s disease, which develop during these transition periods and may represent exaggerated or aberrant maturation processes.
AMPAR Trafficking and Memory
Many studies have implicated synaptic plasticity as a cellular mechanism of learning and memory. Regulated trafficking of AMPA-type glutamate receptors has emerged as a key effector mechanism for expression of synaptic plasticity.
We have found an intriguing age-related requirement for two proteins, PICK1 and KIBRA, which function in a complex to regulate AMPAR trafficking, synaptic plasticity, and learning or memory. Mice lacking either PICK1 or KIBRA show adult onset impairment in hippocampal synaptic plasticity, in spite of missing these proteins throughout development.
Interestingly, alterations in PICK1 function or expression have been linked to schizophrenia, a neuropsychiatric disorder that typically emerges in adolescence or adulthood; KIBRA polymorphisms have been linked to the risk for late-onset Alzheimer’s disease.
We are using these two independent lines of mice to investigate the molecular bases of age-related changes in synapse function, learning, and memory.
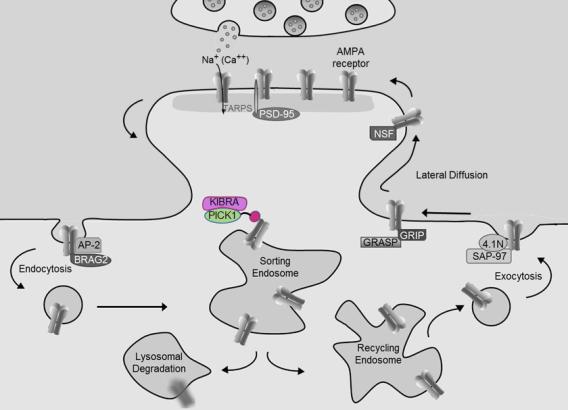 Regulation of dynamic glutamate receptor trafficking
Regulation of dynamic glutamate receptor trafficking