Targeting persister cells in rhabdomyosarcomas
Rhabdomyosarcoma is the most common soft tissue sarcoma in children and young adults, affecting hundreds of families in the U.S. each year. Current treatments - chemotherapy, surgery, and radiation - have not significantly improved survival in over 30 years. For patients with refractory or relapsed diseases, survival drops to ~20%. To make progress, we must understand why these cancers resist treatment.
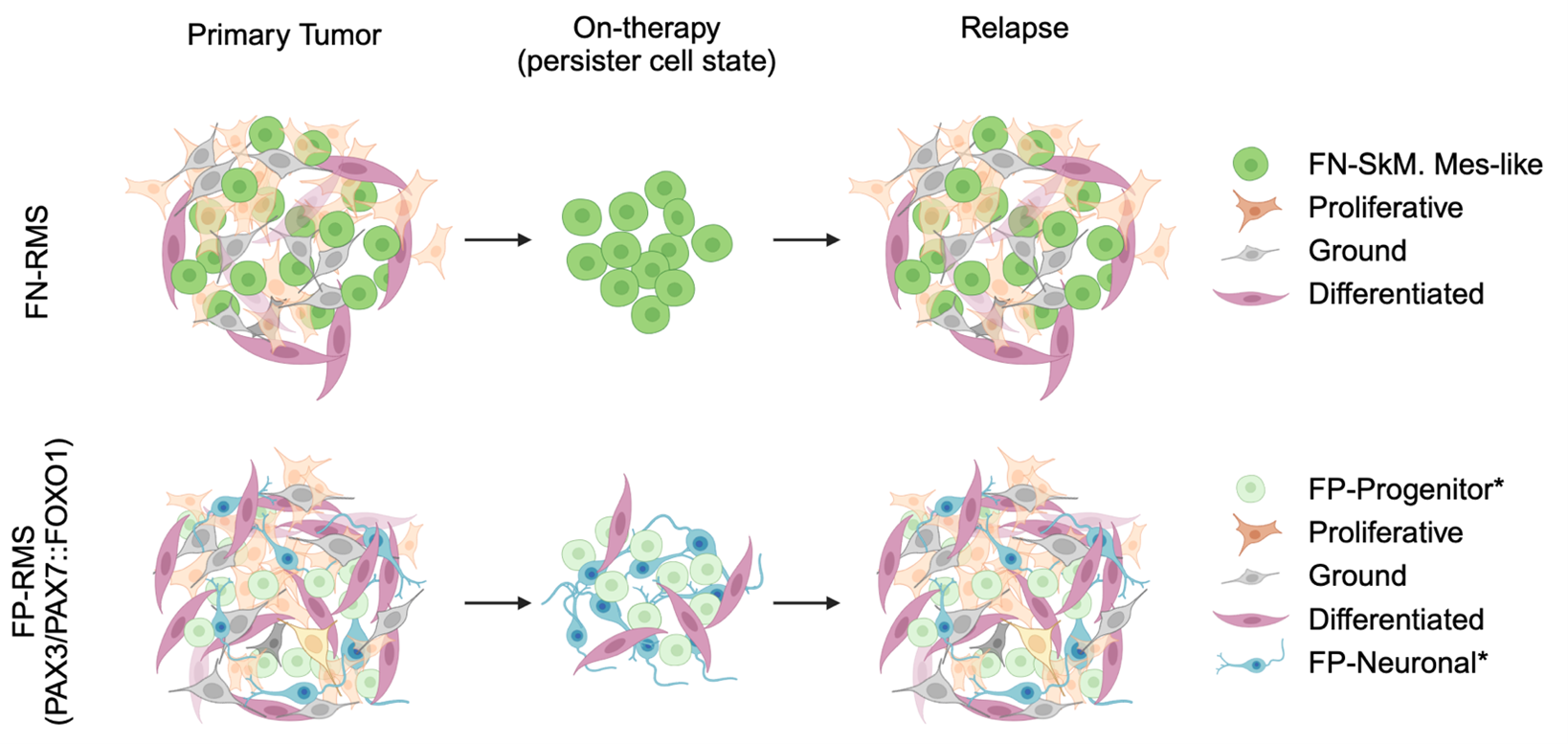
Using single-cell RNA sequencing of tumors from rhabdomyosarcoma patients, we identified a rare subpopulation of “persister cells.” These cells survive chemotherapy and later “wake up”, transitioning into fast-dividing tumor cells that cause relapse. However, how these persister cells endure treatment and re-enter growth remains unclear.
We propose to track these persister cells over time - before, during, and after therapy - using advanced tools such as barcode tracing and single-cell multi-omics. With unique barcodes, we can follow the fate of individual cells, while profiling their gene activity and epigenetic state. Computational modeling will reveal the regulatory network that allows persister cells to 1) tolerate therapy and 2) re-initiate tumor growth.
Importantly, we have found that persister cells emerge regardless of the tumor’s genetic background, suggesting that new therapies must directly target this population. By uncovering the molecular “survival strategies” of persister cells, our work aims to develop the first therapies that stop these dangerous cells before treatment begins - bringing new hope for children with rhabdomyosarcoma.
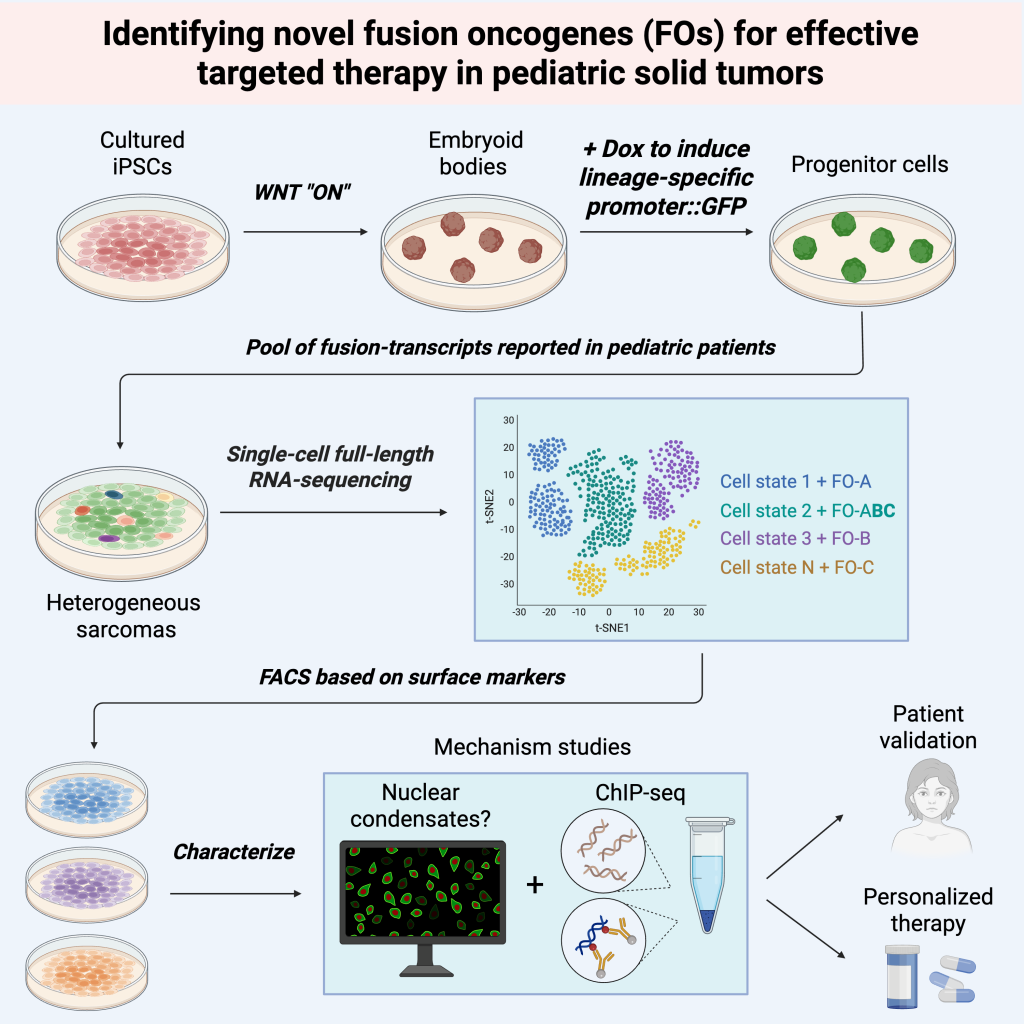
Identification of novel fusion oncogenes in pediatric solid tumors
Fusion transcript events contribute to one facet of tumor heterogeneity in pediatric solid tumors, with subsets of them driving pediatric sarcomas and resulting in poor prognosis. Well-characterized examples include EWSR1-FLI1 in Ewing sarcoma, PAX3/PAX7-FOXO1 in rhabdomyosarcoma, and SS18-SSX in synovial sarcoma. Additional fusion transcripts have been reported in advanced sarcoma cases yet remain poorly understood. Therefore, our overarching goal is to identify novel fusion oncogenes to provide the foundation to develop novel targeted therapies in these pediatric tumors. We will use human induced pluripotent cells to identify novel fusion oncogenes in pediatric sarcomas, with the goal of elucidating their molecular function in driving malignancy. Next, we aim to understand these fusion molecules and then devise novel targeted therapies to improve the outcomes of pediatric cancer patients who have very dismal prognosis associated with these fusions.
Precision immunotherapy for pediatric cancer patients
Treatment outcomes for children / young adults with advanced solid tumors have stagnated over past decades, with a median survival of 20–30%. This is manifested by the lack of effective targeted therapies, largely due to the high degree of tumor heterogeneity from these pediatric patients—evidenced by over 150 distinct subtypes, each affecting a small number of populations.
In the long term, we hope to be part of the team to bring precision medicine to individual patients with a rare and understudied type of cancer. With easy access to next-generation sequencing, single-cell sequencing, and spatial transcriptomics, patient tumors could be molecularly profiled even at the diagnosis stage with a minimal amount of tumor samples. With careful assessment and AI-guided prediction, personalized immunotherapy could be designed and made available to patients, tailored toward individual patients.