Project title: Uncovering Regulators of Cellular Phosphate Homeostasis via Genome-Wide Forward Genetic Screening.
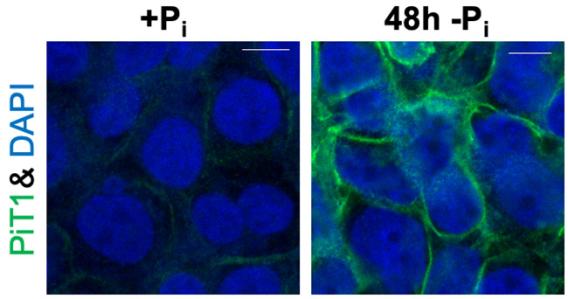
Phosphate starvation (-Pi) leads to striking induction of PiT1/SLC20A1 phosphate transporter protein abundance at the plasma membrane.
My early research focused on muscle energy metabolism. During my Endocrinology fellowship, I became intrigued by a largely unexplored area of the metabolism field: cellular phosphate metabolism in metazoans. Despite phosphate’s central roles in many essential biological processes – including ATP-based energy storage, DNA and RNA structure, and bone formation – our understanding of how individual cells regulate their phosphate levels remains limited. Both phosphate excess (e.g., in chronic kidney disease) and phosphate deficiency (e.g., in many cancers) are linked to significant morbidity and mortality.
To address these knowledge gaps, we study the regulation of PiT1/SLC20A1, a ubiquitous plasma membrane phosphate transporter that is massively induced under phosphate starvation. Inappropriate PiT1 upregulation has been implicated in several diseases including malignancies and vascular calcification, yet the mechanisms controlling its abundance are poorly understood. Our lab investigates loss of which genes blunts PiT1 induction during phosphate starvation and conversely, loss of which genes leads to inappropriate PiT1 induction under phosphate-sufficient conditions.
Our findings highlight the ESCRT-lysosome axis as a key pathway for PiT1 degradation, acting as a negative regulator of its protein levels in phosphate-sufficient environments. These insights - along with findings from our phosphate-starved screen - have led to the identification of several candidate regulators of PiT1 and, more broadly, cellular phosphate homeostasis. They form the foundation of ongoing research in the Zechner lab.
Relevant publications:
Zechner C, Henne WM, Sathe AA, Xing C, Hernandez G, Sun S, Cheong MC. Cellular abundance of sodium phosphate cotransporter SLC20A1/PiT1 and phosphate uptake are controlled post-transcriptionally by ESCRT. J Biol Chem. 2022 Jun;298(6):101945. doi: 10.1016/j.jbc.2022.101945. Epub 2022 Apr 18. PMID: 35447110; PMCID: PMC9123275.
Zechner C, Rhee EP. Phosphate sensing in health and disease. Curr Opin Nephrol Hypertens. 2024 Jul 1;33(4):361-367. doi: 10.1097/MNH.0000000000000984. Epub 2024 Apr 2. PMID: 38572729.
Goals of the Zechner lab:
- Elucidate mechanisms regulating PiT1/SLC20A1 protein abundance and trafficking.
- Identify cellular machinery responsible for phosphate sensing.
- Discover adaptive responses to both phosphate deficiency and excess.
Current and Past Research Support:
NIGMS R35 MIRA Award
NIDDK K08 Award
UT Southwestern
(Pak Family Center & Bone Initiative, Division Chief-Nominated Pilot & Feasibility Projects in Biochemical and Clinical Research)