P53 signature - latent precancer of type II endometrial cancer
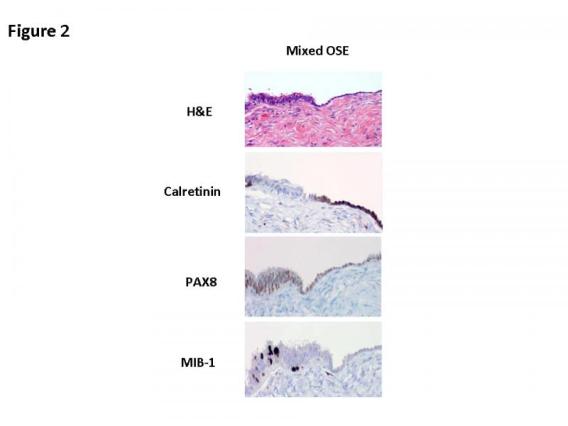
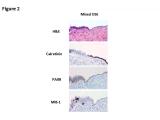
Based on our previous studies, it is concluded that alteration of p53 occurs not only in well developed ESC, but also in its early form (endometrial intraepithelial carcinoma, EIC) and precancerous form (endometrial glandular dysplasia, EmGD). We have recently identified a group of benign-looking endometria with p53 overexpression, which we designated as “p53 signatures”. Strictly speaking, “p53 signatures” represent those endometrial epithelial cells, with either glandular or surface growth patterns, which are morphologically unremarkable but display diffuse and strong p53 nuclear staining. The following panel contains representative pictures of p53 signatures. The upper left shows surface endometrium is strongly positive for p53 nuclear overexpression, but in the corresponding H&E section (upper right), these p53 positive cells are morphologically unremarkable. Similarly the single p53 positive gland in lower left panel shows no morphologic changes (lower right).
The p53 signatures were specifically associated with ESC, being frequently found in the benign-appearing endometrium adjacent to ESC and only rarely in the endometrium adjacent to endometrioid carcinomas or in non-cancerous uteri. Our recent study showed that 42% of the p53 signature samples showed at least one p53 gene mutation. There were 8 ESC uteri with p53 signatures showing p53 gene mutations. And high concordant p53 mutations present in p53 signatures, EmGD, EIC/ESC within the same uterus. The finding of identical p53 mutations provides further evidence of a probable shared lineage between those lesions, and suggests that the p53 signature epithelia/glands are likely latent precancers
Endometrial serous carcinoma (ESC), previously called as uterine papillary serous carcinoma (UPSC), arises from resting endometrium and is independent of estrogen stimulation. It has been notorious for its aggressive behavior and dismal prognosis with the outcome of a disproportionate number of endometrial cancer deaths although it comprises only about 10-15% of all endometrial cancer. Since its etiology is virtually unknown, it is difficult to manage the patients with ESC. However, identifying precancerous lesion(s) of such disease may provide a good opportunity for early diagnosis and better management.
Serous endometrial intraepithelial carcinoma (EIC) was first proposed as a putative precancer of ESC in 1992 [1] and formalized in 1995 [2], which was defined morphologically as the replacement of endometrial glandular epithelia by frankly malignant cells identical to that of ESC cells without myometrial or stromal invasion. Serous EIC and ESC were assumed to arise in benign atrophic endometrium (AE). How AE “suddenly” becomes serous EIC? This phenomenon was interpreted by many gynecologic pathologists as “De Novo” process. Based on
Dr. Zheng’s understanding, “De Novo” is usually the term people use to explain some observations without in depth understanding. Apparently, there is something missing between benign AE and serous EIC. In 2000, Wenxin Zheng and his colleagues at Yale Pathology made a hypothesis that there must be an entity linking AE and serous EIC. Morphologically, the endometrial glandular cells of this entity should present the degree of nuclear atypia falls short of serous EIC but apparently more than AE and its related reactive changes.
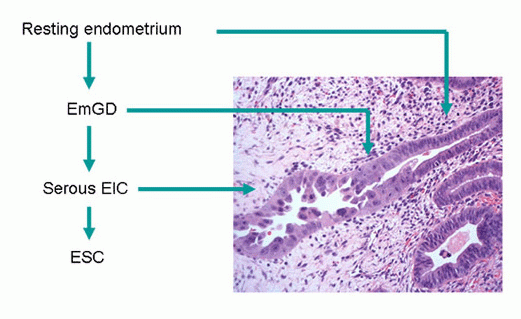
Based on this assumption, Sharon Liang, Dr. Zheng’s gynecologic pathology fellow at Yale and Dr. Zheng started to carefully look for those lesions from ESC uteri. They soon found that there was indeed such entity present in ESC uteri. This was defined as endometrial glandular dysplasia (EmGD). The first 2 articles, one morphologic study and the other molecular study, were published in 2004 in International Journal of Surgical Pathology[Zheng and Liang].
There were several side points worth to mention. One was regarding the terminology EmGD. Zheng’s group originally would like to use endometrial intraepithelial neoplasia (EIN), but the EIN term has been used by Mutter et al for type I endometrial precancers. Therefore, the term EmGD was chosen to describe the new entity. It seems that they may have a chance to unify all endometrial neoplastic process into a single classification system [Click here]. The other was the entity recognition in the gynecologic pathology field. There is a group of pathologists who still consider EmGD is part of the spectrum of serous EIC.
Dr. Zheng once had a conversation with a representative of that group when he presented EmGD story in USCAP meeting in 2003. That representative admitted that they saw “EmGD” lesions back to early 1990s but failed to define it when they described EIC at that time. This was mainly because they did not know how to put all the pieces together to resolve this puzzle.
If EmGD is the precancer of ESC, then what is serous EIC and what is the relationship among EmGD, serous EIC and ESC. Based on clinical findings that serous EIC is commonly associated with extrauterine disease ranging from 33% to 67% and morphologic findings that serous EIC cells are identical to those ESC cells, Zheng’s group further proposed that serous EIC is an early serous carcinoma rather than a precancer of ESC [Zheng and Schwartz]. It is likely that serous EIC develops from EmGD then to ESC. Interestingly, we found that serous EIC or ESC and EmGD are frequently associated with resting endometrium (RE) rather than with AE only. RE includes AE, weakly proliferative endometrium and proliferative endometrium. Not infrequently,women in postmenopausal age are taking hormones as a replacement, which correspond to the proliferative status of the endometrium.
One clinical impact for this finding is that a pathologist should not be afraid of making the diagnosis of ESC when he/she sees the background endometrium is proliferative. Since type II endometrial cancer consists of serous as well as clear cell carcinomas and many ESC uteri having areas of clear cell component or vice versa, Zheng’s group studied uteri with clear cell carcinoma. Luckily, lesions ofclear cell EmGD and clear cell EIC were identified and they are commonly associated with clear cell carcinoma [Fadare]. More recently, Zheng’s group found that lesions of EmGD have frequent p53 gene mutations [Jia]. Furthermore, latent precancer of ESC, a step earlier than EmGD has been just described [Zhang].
 Endometrial serous carcinoma (ESC)
Endometrial serous carcinoma (ESC) In 2006, a definition of “precancer” in the majority of human organ sites was established by the National Cancer Institute-sponsored consensus conference [Link]. Intriguingly, EmGD turns out to meet all of the criteria included in the definition and fulfill the role to be the putative precancer to ESC [Yi and Fadare].
First, a precancer is different from the normal tissue from which it arises. EmGD is an identifiable entity with the nuclear atypia exceeding that of adjacent normal resting endometrium [Zheng].
Second, a precancer shares some but not all molecular and phenotypic properties that characterize the cancer. EmGD often develops in multicentric sites that are noncontiguous with the main tumor mass of ESC but often associated with serous EIC. No direct transitions are observed between EmGD and ESC. Cytologically, the degree of nuclear atypia in EmGD falls short of serous EIC, which is the main difference between EmGD and serous EIC [Zheng]. Molecular evidences show that the load of alternations increased from resting endometrium to EmGD to serousEIC and/or ESC as evidenced in LOH of TP53 and chromosome 1p [Liang], mutation rate of p53 [Jia] and IHC index of p53, MIB-1 and another new cytoplasmic marker called IMP3 [Zheng].
Third, the cancer arises from the cells within the precancer. In this study, at least one identical p53 gene mutation was found in lesions of EmGD and ESC or serous EIC within the same uterus in all the 6 cases. Such a high concordance demonstrates a monoclonal growth of ESC from precancer cells.
Fourth, there are methods by which precancer can be diagnosed. The diagnostic criteria have been described before. It is mainly based on morphologic changes under microscope by excluding serous EIC or clear cell EIC. IHC analysis of p53, IMP3 and MIB-1 is helpful when obscure judgment is encountered. More objective computerized karyometric analysis of H&E stained slides is being carried out to improve the reproducibility.
Finally, those precancer patients are associated with an increased risk of cancer. In our retrospective study, a 9-fold increased risk of developing ESC is observed in those with EmGD diagnosis [Zheng].
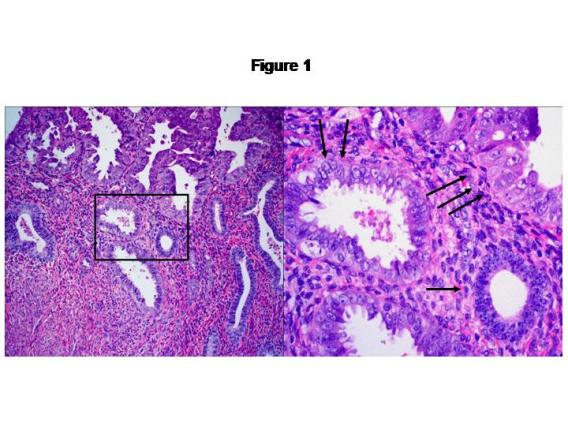
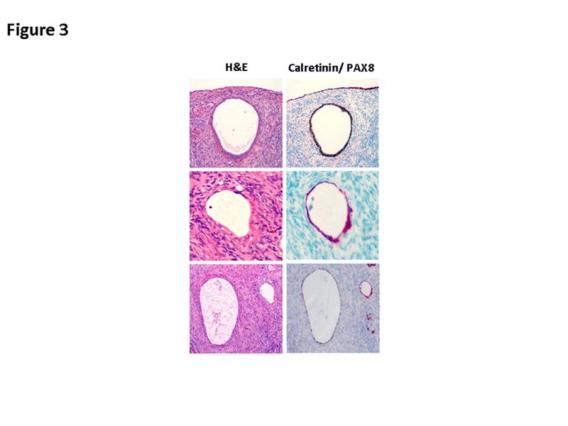
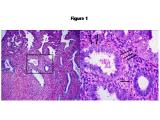
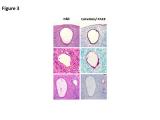
On the basis of our studies and available clinicopathologic evidence in the past years, we advocate a model for endometrial serous carcinogenesis. In this model, endometrial serous carcinoma arises predominantly in the resting endometrium, manifesting first as p53 immunoreactive, morphologically normal endometrial cells (p53 signature), evolving to endometrial glandular dysplasia (which is the first morphologically identifiable precursor lesion), then to serous endometrial intraepithelial carcinoma (a carcinoma with a noninvasive growth pattern in the uterus but which is frequently associated with extrauterine disease), and finally into fully developed serous carcinoma. The representative pathologic pictures for ESC and its precursors are illustrated in Figures 1 to 3.
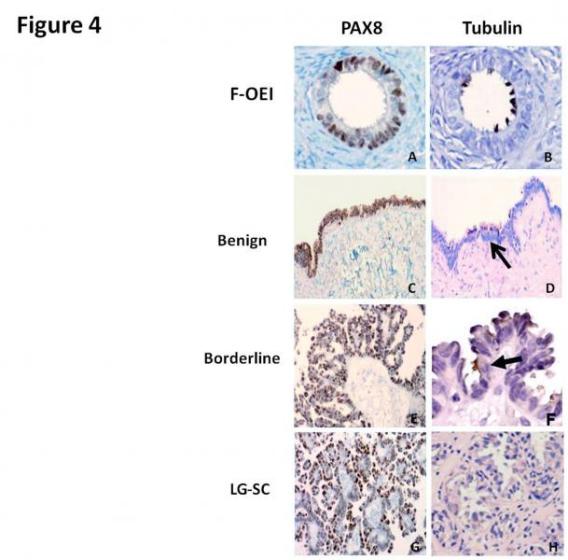
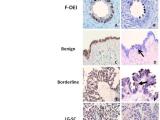
There have been many molecular alterations in the evolution of the tumor. Some of these molecular events are also present in its putative precursor lesions, such as p53 and BRCA mutation, HER2/neu amplification, and the molecular changes of IMP3 and Nrf2, etc (Figure 4).
A dualistic model has been developed in endometrial carcinogenesis based on the clinico-pathological observations and molecular evidence, which provides a valuable framework for the study of various aspects of endometrial carcinogenesis and for the potential development of therapeutic modalities that are pathway specific.
One aspect of cancer prevention is the recognition of morphologically distinctive precursor lesions or “precancers”, so that a therapeutic intervention can be administered prior to the development of the well-developed malignancy. However, sometimes conclusions are hard to draw by comparing the so-called “precancer” related data, basically due to the problem of ever-evolving nomenclature of endometrial precancers.
Among endometrial carcinomas, endometrioid carcinoma is the most frequent and is the most extensively explored subtype, with the most various terminologies created for precursor descriptions.
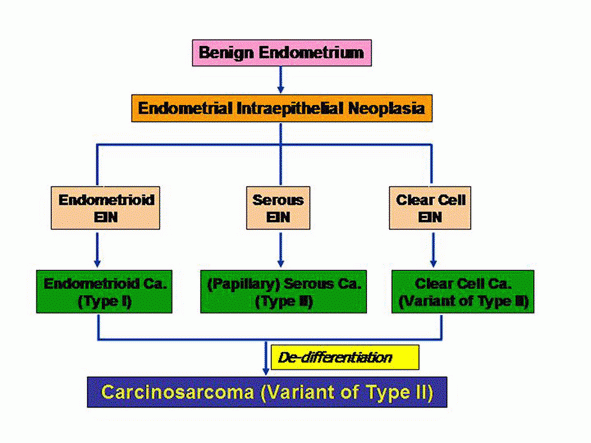
We propose a nomenclatural system for the putative precursor lesions that represents an expansion of the definition of endometrial intraepithelial neoplasia (EIN) designation and which can potentially be applied to all major histologic types of endometrial neoplasms. All precursor lesions will be designated EIN but always with a prefix that identifies the histologic subtype of the invasive cancer that they are supposedly a precursor of.
In the context of endometrioid lesions, the EIN system of Mutter et al will be adopted, with a distinction been made between benign endometrial hyperplasia and strictly defined EIN; the latter will always be referred to as Endometrioid EIN.
- In the context of serous lesions, the terms EIC or serous EIC will be discontinued; EmGD lesions will be designated serous EIN.
- For clear cell lesions, the putative precursor lesion previously described will be referred to as Clear cell EIN. This is illustrated in the following graph:
- Prospective studies with follow-up information are needed to define relative risk factors involved in the progression from EmGD to a full-blown ESC and further develop optimal clinical management.
- Validations from multiple institutions should be carried out to confirm the previous findings and make this diagnose feasible worldwide.
- The early recognizable entity EmGD provides an opportunity to identity molecular alterations in the initiation of ESC. Stem cells with epigenetic alterations might be the source of the malignant transformation, which is currently being pursued in our laboratory.
- A p53 protein based early detection assay needs to be developed for ESC. This assay can be used initially for those high risk patients, then apply to general use when appropriate.
- Animal models with genetic manipulations such as p53, IMP3, Nrf2, and BRCA etc to further understand initial genetic alterations in the process of endometrial serous carcinogenesis.