Immunotherapy harnesses the immune system to attack tumor cells in the same way that it attacks foreign pathogens, and it holds great promise for advancing cancer treatment, especially when combined with other therapies, such as radiation or chemotherapy. Our laboratory is studying the interactions between radiation and the immune system from the molecular level – where DNA damage caused by radiation triggers an immune response – to the clinic, where new combinations of radiation therapy and immune agents may yield more effective therapeutic regimens to treat various cancers. We are actively working to understand the mechanisms and processes involved in reliable immune response after genotoxic cancer therapeutics as well as test combination therapies in the clinic.
Genotoxic cancer therapy agents kill cancer cells mainly by damaging their DNA, which affects these cells’ ability to replicate and can lead to cell death. However, the DNA damage caused by these agents also sets in motion a host of molecular factors that respond to and try to repair this damage, as well as factors that activate and regulate the immune system. Developing new therapeutic agents and combinations that can exploit the immune response to genotoxic cancer therapeutics requires understanding the complex molecular interplay among these factors.
Specifically, our lab studies the molecular factors that respond to and repair DNA damage caused by cancer therapeutics and other genetic or environmental influences, focusing on how these factors interact with, trigger, or suppress the immune system. Through these studies, we seek to identify novel immunomodulatory drugs that can exploit DNA repair and DNA damage response factors within cancer cells to trigger immune signaling after irradiation and chemotherapy and recruit immune cells to the tumor microenvironment to eradicate cancer cells.
Directing the immune system to attack cancer cells but ignoring normal, non-cancerous cells requires identifying tumor-specific neoantigens or antigens that form on certain types of cancer cells, but not on normal cells, for immune cells to target. Our lab is currently investigating neoantigens specific to BRCA1-mutant breast cancer, in collaboration with Dr. Chao Xing and Dr. Tao Wang from the Department of Clinical Science, to locate potential targets for new vaccines. “Our research will not only help us to develop novel vaccines against cancer-specific neoantigens but also redirect immune cells to the tumor microenvironment to eradicate metastatic cancer cells. The clinical potential of our efforts is substantial: not only could they improve treatment for BRCA1-mutant breast cancer, but they may even help prevent it through vaccination.
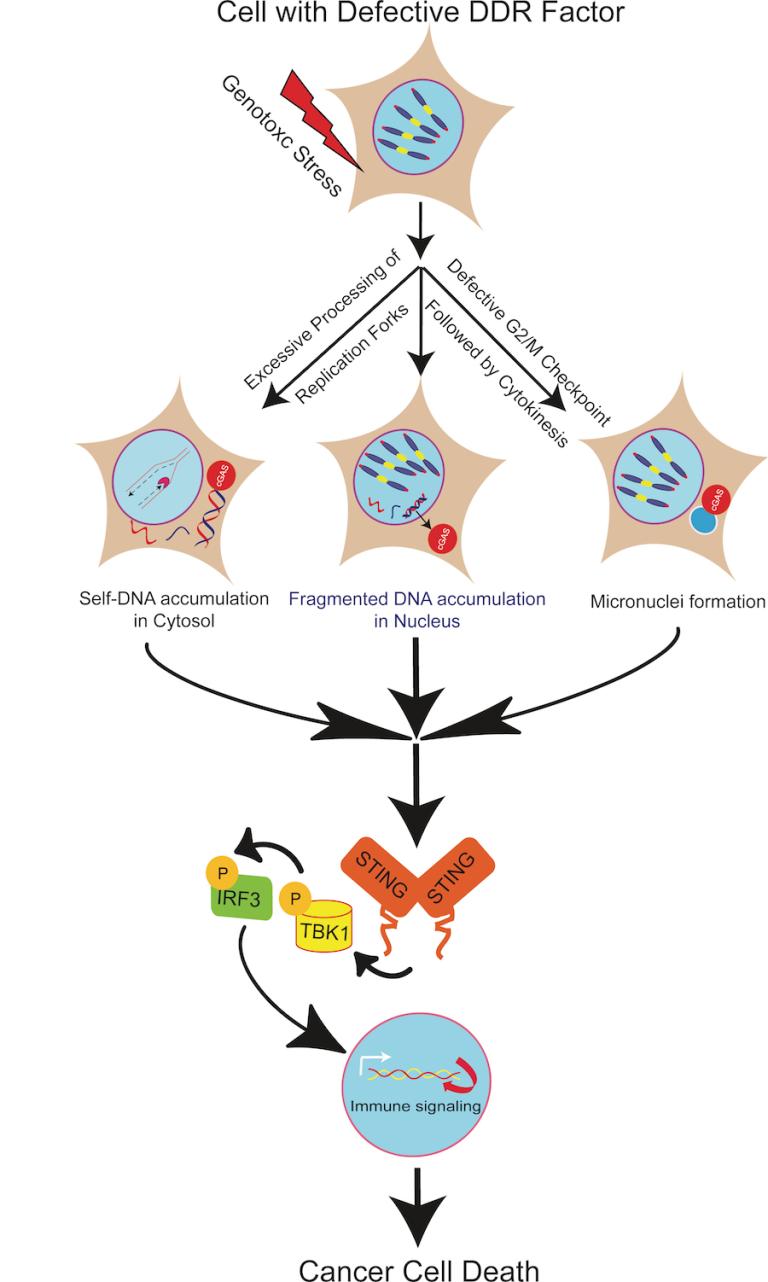 Triggering and redirecting innate immune signaling to eradicate cancer cells
Triggering and redirecting innate immune signaling to eradicate cancer cells Reference: Bhattacharya et al. RAD51 interconnects between DNA replication, DNA repair and immunity. Nucleic Acids Research. 2017. DOI:10.1093/nar/gkx126.