As part of a collaboration with Beatriz Fontoura (Cell Biology) and Meg Phillips (Pharmacology), we synthesized the most potent DHODH-inhibitor reported to date (IC50 1 nM) that inhibited viral replication of VSV and WSN-Influenza (EC50 2 nM and 41 nM), and solved the X-ray structure of human DHODH bound to this potent novel inhibitor.
In collaboration with Luis Parada (MSKCC), we developed several different novel small molecule collections from four different scaffolds with different modes-of-action that potently and selectively kill tumor-derived neuronal stem cells for the potential treatment of glioblastoma. One series, termed Gboxins, was found to specifically inhibit oxidative phosphorylation via inhibition of complex I of the respiratory chain. Another series was found to selectively inhibit the growth of Glioma Stem-Like Cells (GSCs). Mode-of-action studies in collaboration with Deepak Nijhawan have led to the identification of lanosterol synthase as the molecular target of these compounds. Collaborative studies with the McBrayer lab are underway to investigate the translational potential of optimized brain-penetrable analogs.
In a collaboration with Jerry Shay (Cell Biology) aimed at identifying compounds with selectivity for colon cancer cells with truncating mutations in the APC gene, we initiated a medicinal chemistry program that led to an analog collection of piperidinyl sulfonamides (>200), that kill truncating APC-expressing colon cancers with exquisite in vitro selectivity versus any other cell lines that lack these mutations (picomolar versus 10 µM!). This selectivity was maintained in vivo (xenografts) and a non-toxic orally available analog successfully prevented the development of colon cancer in a GEM mouse model of familial adenomatous polyposis. Medicinal chemistry enabled biochemical target identification studies in collaboration with the Nijhawan lab that led to Emopamil Binding Protein (EBP, a sterol isomerase) as the molecular target of TASINs (Truncated APC-Selective Inhibitors).
Other collaborative synthetic and medicinal chemistry-oriented projects in our lab also include structure-based design of orexin-receptor agonists for narcolepsy and positive allosteric modulators of the dopamine receptor (with Dan Rosenbaum).
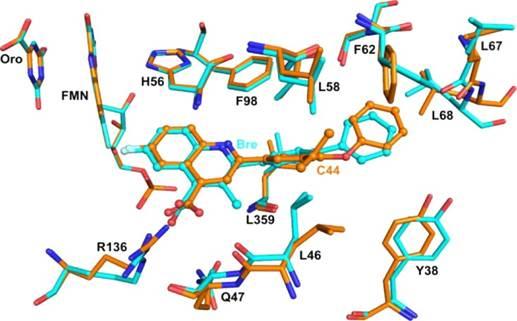 X-ray structure of human DHODH bound to a novel inhibitor – ACS Med. Chem. Lett. 2013, 4, 517
X-ray structure of human DHODH bound to a novel inhibitor – ACS Med. Chem. Lett. 2013, 4, 517