How do cells store and organize their fat & maintain organelle lipid quality?
Life presents energetic and metabolic challenges. To ensure their survival in a constantly changing environment, cells developed ways to store excess nutrients in the form of high-energy triglycerides (TG) kept within lipid droplets (LDs). Beyond their role in energy homeostasis, LDs also help cells buffer lipotoxicity (see recent review Henne & Cohen, Nature Reviews Molecular Cell Biology, 2026). Organelles must also encode lipid quality control (LQC) factors to suppress lipid damage such as lipid peroxidation that can drive organelle dysfunction. The endoplasmic reticulum (ER) is one such organelle that must encode LQC factors, and we recently characterized an enzyme (APMAP) that protects ER membrane lipids from damage (see below and Paul, Developmental Cell, 2025).
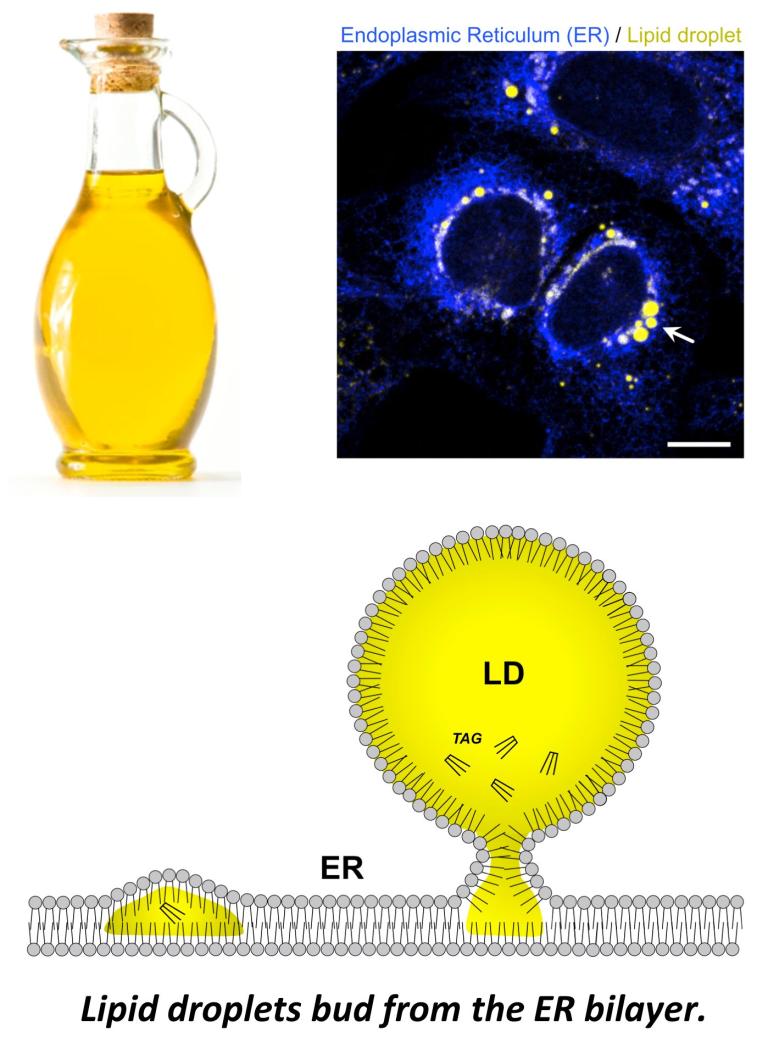
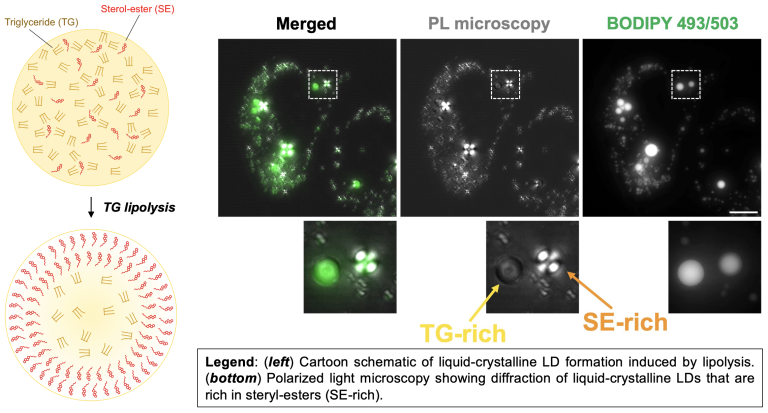
Lipid droplets can contain TG and CE, which influences LD biophysical properties
The hydrophobic lipid core of LDs can contain triglyceride (TG) as well as cholesteryl-ester (CE). At body temperature, CE is not soluble and forms liquid-crystalline deposits that contribute to cardiovascular disease. Our lab studies how LDs influence the solubility of CE, and recently found that TG can dissolve CE in LDs, keeping it soluble. Metabolic cues like lipolysis that mobilize TG in LDs can thus influence CE biophysical properties. LDs with high CE compared to TG un phase transition into liquid-crystalline LDs (see our paper Rogers, et al, JCB, 2022; see Rogers, JOVE, 2023 video protocol). The TG:CE ratio within LDs thus has a significant impact on cholesterol metabolism and human health.
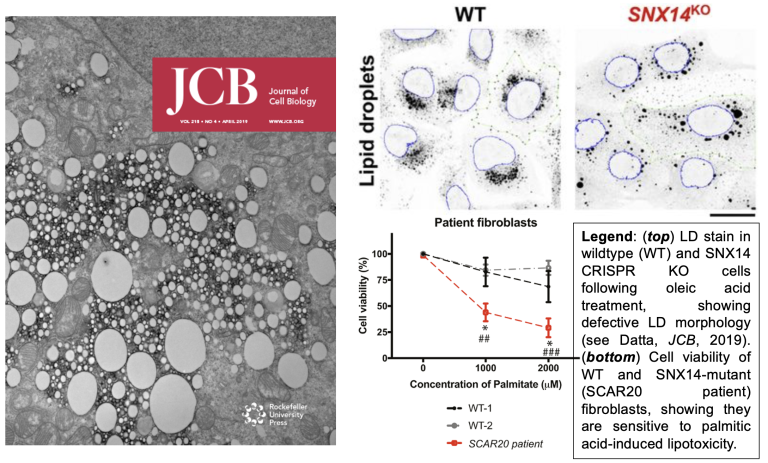
Inter-organelle tethers influence lipid storage and neurodegenerative disease
Cells encode lipid transport proteins that help buffer lipid storage and suppress lipotoxicity that can drive disease. Our lab has characterized a family of proteins (the SNX-RGS proteins, and SNX14 in particular) that promote lipid storage and organelle lipid homeostasis. We find that SNX14, mutations in which cause the cerebellar ataxia disease SCAR20, is a lipid transporter that can promote lipid storage in LDs and suppresses lipotoxicity (Bryant, HMG, 2018; Datta, JCB, 2019; Datta, PNAS, 2020; Paul, FCDB, 2022). SNX14 is highly conserved, and yeast ortholog MDM1 also promotes lipid homeostasis by promoting lipid storage in LDs (see Hariri, EMBO reports, 2017; Hariri, JCB, 2019).
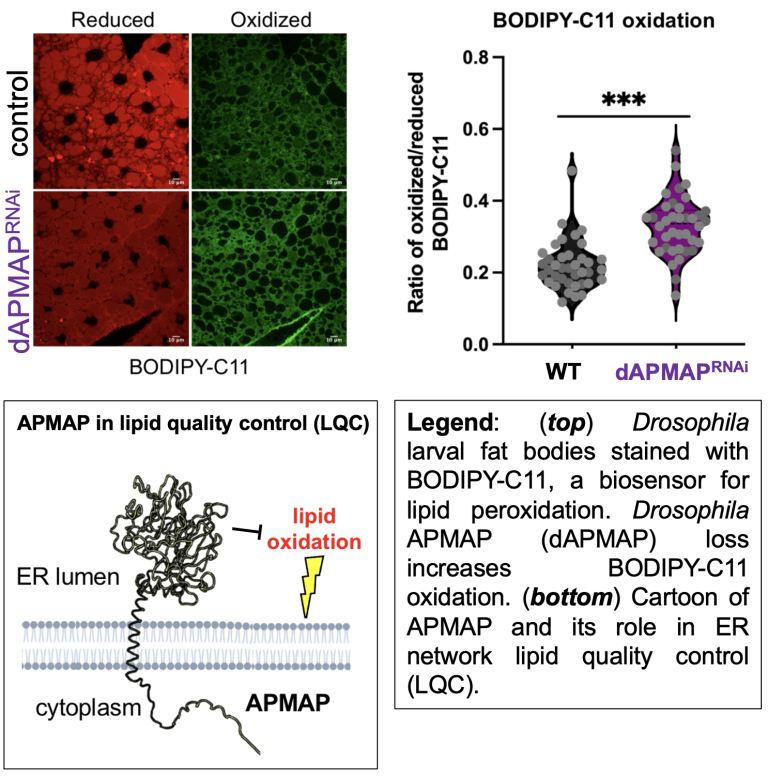
Lipid peroxidation at the ER network can drive cell toxicity
The ER maintain a highly oxidative lumen to support oxidative protein folding. However, a pervasive question is how the ER maintains its own membrane lipid quality and suppresses lipid peroxidation events that can lead to ER dysfunction. We recently identified a new ER localized lipid quality cotrol (LQC) enzyme called APMAP that suppresses ER lipid oxidation (Paul, Developmental Cell, 2025). APMAP contains an arylesterase domain similar to paraoxonase-1 (PON1), which suppresses the accumulation of oxidized lipoproteins in the blood. Similarly, we find APMAP acts intracellularly to suppress reduce lipid oxidation. APMAP-depleted human or Drosophila cells manifest ER stress and elevated lipid peroxidation.