We are interested in the relationship between cellular lipid metabolism and organismal aging. To mechanistically understand this relationship, we utilize the model organism Drosophila melanogaster (the fruit fly).
Many recent studies indicate that nutrient stores in the form of triglycerides (TAG, or TG) within lipid droplets (LDs) are important to maintain organismal energy homeostasis. During aging, these LD stores can act as a metabolic buffer that modulates changes in dietary homeostasis. We and others have found that promoting TAG storage in Drosophila adipocytes can promote healthy aging and resistance to metabolic stress. Recently we identified a novel inter-organelle tethering protein, Snazarus (Snz), that promotes TAG storage in Drosophila adipose tissue when over-expressed (Ugrankar, et al, Developmental Cell, 2019).
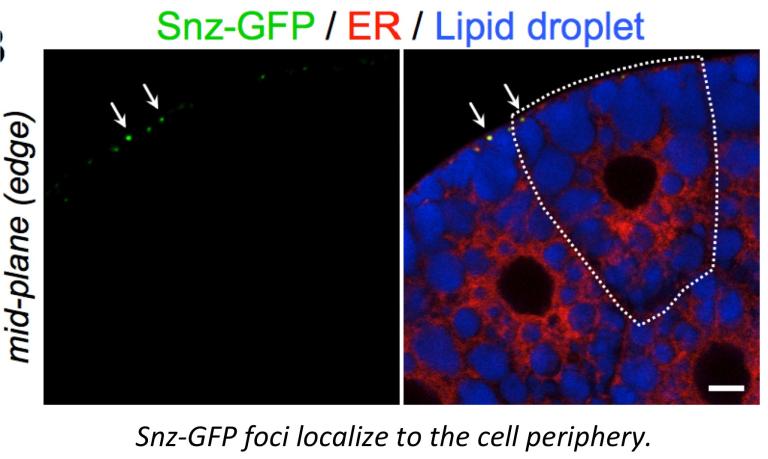
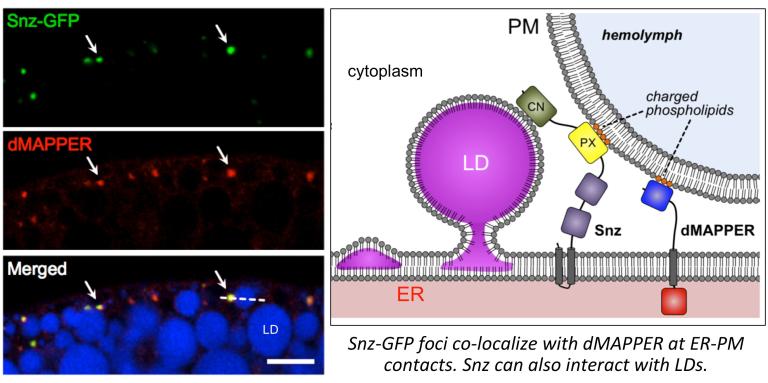
The Drosophila protein Snazarus (Snz) is a key regulator of this energy balance in adipocytes. We find that Snz is an ER-associated protein that interacts with LDs near the cell plasma membrane (PM) at ER-PM contact sites (labeled with the ER-PM biomarker dMAPPER). Snz interacts with LDs and promotes TAG storage. We are currently investigating how Snz functions at ER-PM contact sites and promotes TAG storage.
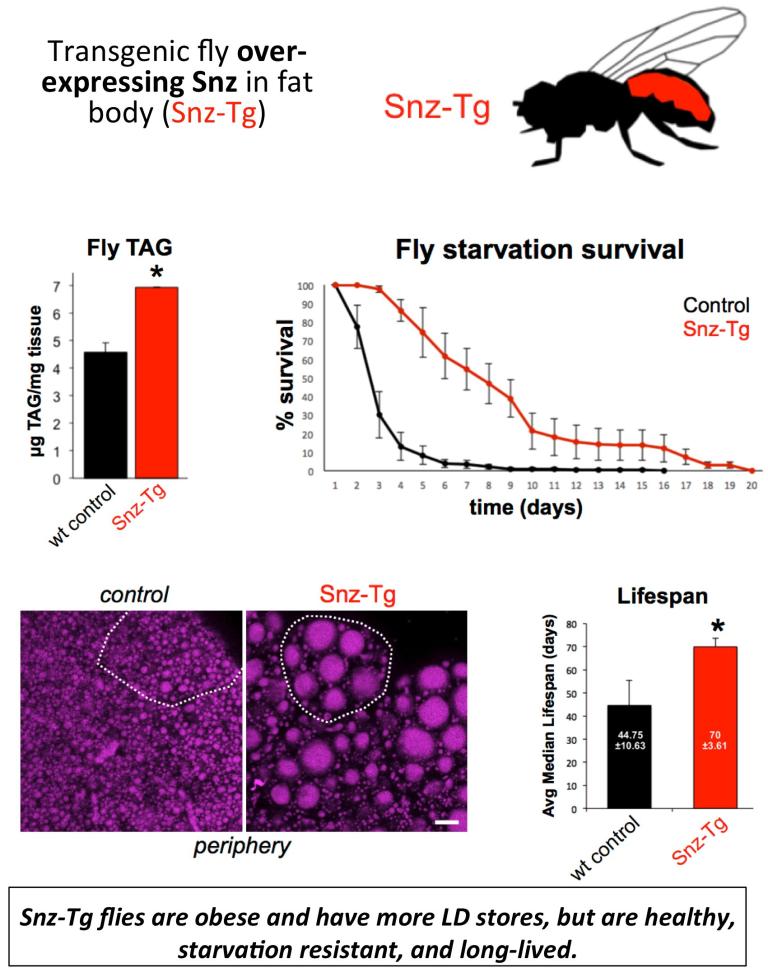
Since Snz interacts with LDs and promotes TAG storage, we interrogated whether modulation of Snz expression in the adipocyte tissue (the fly fat body) would affect aspects of organismal homeostasis. We generated a transgenic fly over-expressing Snz in the fat body (Snz-Tg). Intriguingly, the Snz-Tg fly has elevated TAG stores but also exhibits an extended lifespan and starvation resistance. We are currently investigating how elevated TAG stores in adipocytes can promote organismal homeostasis and healthy aging.
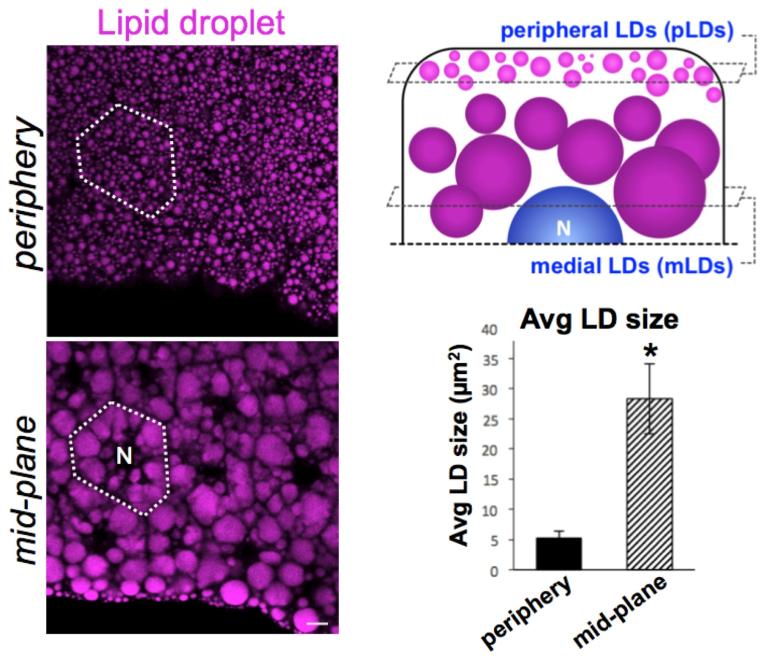
How are lipid droplets organized within Drosophila adipocytes?
We are also interested in how Drosophila adipocytes organize their fat stores. Remarkably, we find that Drosophila adipocytes maintain two distinct LD sub-populations: a peripheral LD (pLD) population near the cell surface and larger medial LD (mLD) population near the nucleus. These LD sub-populations appear to be maintained by distinct cellular mechanisms, suggesting specialized functions for each LD sub-population. We are currently investigating the functions of these LD sub-populations in cellular and organismal physiology.