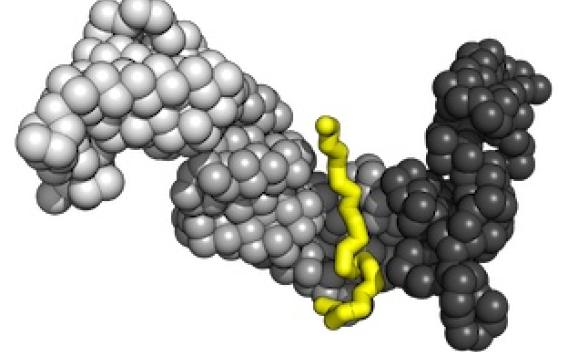
Protein misfolding is a key element of Alzheimer’s and other neurodegenerative diseases.
Our lab seeks to uncover the structure-function relationship of macromolecules involved in protein misfolding.
We study how conformational changes lead proteins to self-assemble into larger structures, and how cofactors tune the formation of these structures. We believe this work will lead to breakthroughs in understanding and treating neurodegenerative diseases.
To this end, we are developing strategies that integrate crosslinking mass spectrometry with structural, computational, and biochemical approaches. Using this hybrid approach, we have purified and characterized two distinct forms of tau protein monomer: an inert form, and another that readily self-assembles into large aggregates.
This finding, driven by our novel methods, opens exciting avenues of research and treatment, such as detecting pathological forms of tau in people before they develop symptoms of Alzheimer’s disease or other tauopathies.
Tau Aggregation
We want to understand how tau's inert form transforms into the pathological shape.
Learn about Tau Aggregation
Hybrid Structural Analysis
We use a variety of methods to uncover complex structure-function relationships in intrinsically disordered proteins.
Learn about Hybrid Structural Analysis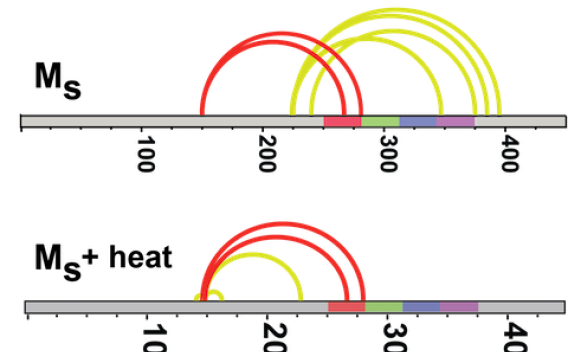
Structure & Aggregation
We focus on tau's "amyloid motif," a sequence of six amino acids that drives tau aggregation and pathology.
Learn about Structure & Aggregation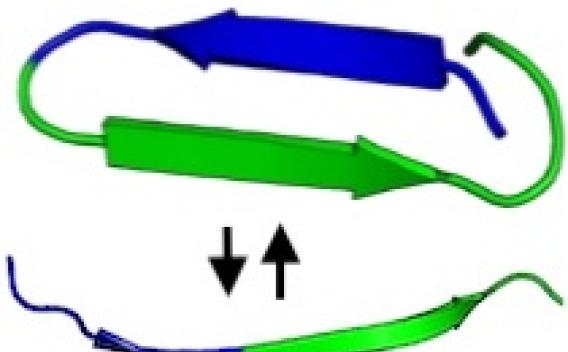
Chaperones & Aggregation
We employ a variety of analytic techniques to uncover the kinetics, structure, and biochemistry of chaperones and amyloidogenic proteins.
Learn about Chaperones & Aggregation