The Liou Lab seeks to understand the principles underlying communication between organelles within mammalian cells. Such communication is essential, not only to maintain cell homeostasis, but also to properly respond to stimulation from the environment. We employ cutting-edge techniques, including live-cell and super-resolution imaging, proximity-labeling proteomics, and reverse genetic screens, to investigate spatiotemporal regulation of signaling events occurring between organelles in physiological and pathological states.
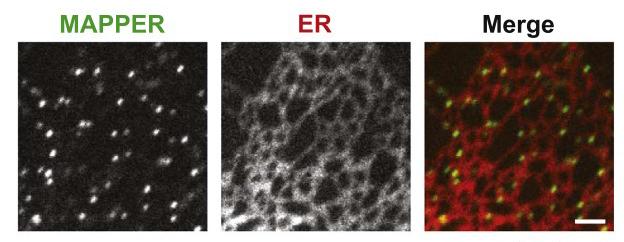
Visualization of ER-PM junctions using ER-labeling or MAPPER
Membrane Contact Sites
Inter-organelle communication can be mediated at membrane contact sites where two organelles are in close contact to each other within 10-30 nm, permitting direct molecular interactions. Contact sites formed between the endoplasmic reticulum (ER) and the plasma membrane (PM), known as the triad or dyad junctions, have been shown to be important for excitation-contraction (E-C) coupling for muscle functions several decades ago. The identification of STIM1 that mediates store-operated calcium entry ( SOCE) at ER-PM junctions revealed the general importance of ER-PM junctions in calcium signaling in all mammalian cells. We aim to understand how ER-PM junctions are formed and regulated by developing genetically-encoded markers and imaging methods to track these junctions in live cells, and by performing proteomic analysis to identify proteins selectively localize at ER-PM junctions. In addition, we are investigating signaling events occurring at ER-PM junctions and their functional significance to cell physiology.
Mapper is available at Addgene now!
Our research has contributed to
- Identification of STIM1 that mediates SOCE at ER-PM junctions
- Characterization of molecular mechanisms underlying STIM1 targeting to ER-PM junctions
- Development of MAPPER that selectively labels ER-PM junctions based on the targeting mechanism of STIM1
- Characterization of the regulation of ER-PM junctions during calcium signaling
- Identification of E-Syt1 and Nir2 that dynamically localize to ER-PM junctions and mediate PI(4,5)P2 replenishment during calcium signaling
- Characterization of molecular mechanisms underlying Nir2 and Nir3 targeting to ER-PM junctions
- Visualization of spatial organization of ER-PM junctions using super-resolution microscopy
- Identification of RASSF4 that regulates the stability and formation of ER-PM junctions
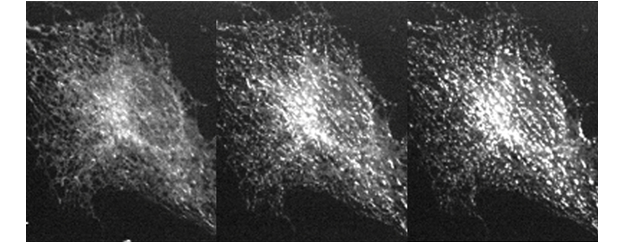
STIM1 translocation to ER-PM junctions after ER calcium is depletedns using ER-labeling or MAPPER
Calcium signaling is important for numerous physiological functions such as lymphocyte activation, muscle contraction, and neuronal transmission. In resting cells, cytosolic calcium concentration is maintained at a very low level by pumps that actively move calcium ions to the extracellular space and to the lumen of endoplasmic reticulum (ER). Following stimulation of many cell surface receptors, calcium is first released from the ER to the cytosol. ER calcium depletion triggers conformational change and oligomerization of the ER membrane protein STIM1, resulting in its translocation to ER-plasma membrane (PM) junctions and activation of the PM calcium channel Orai1. This process is called store-operated calcium entry (SOCE) that brings in calcium from the extracellular space to sustain cytosolic calcium signals, and to refill the ER calcium store following its depletion. Defective SOCE is associated with numerous human diseases, including severe combined immunodeficiency, skeletal myopathy, and neurodegeneration.
Our research has revealed
- STIM1 as the ER calcium sensor for SOCE
- STIM1 translocation to ER-PM junctions when ER calcium is depleted
- The four steps of STIM1 ER-to-PM signaling relay to activate SOCE
- Feedback regulation of calcium signaling by E-Syt1 and Nir2 at ER-PM junctions
- RASSF4 as a positive regulator of SOCE and ER-PM junctions
- The role of EB1 binding in STIM1 translocation to ER-PM junctions and SOCE activation
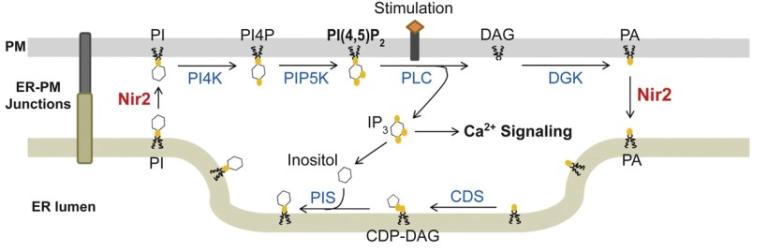
PI cycle with Nir2 mediating PI-PA exchange at ER-PM junctions
Phosphoinositide is a family of phospholipids fundamental to almost all cellular functions. Abnormal phosphoinositide metabolism is linked to numerous diseases including cancer, neuropathy, and myopathy. The level of phosphatidylinositol 4,5-bisphosphate (PI[4,5]P2), a phosphoinositide enriched at the plasma membrane (PM), regulates membrane trafficking, ion transport, and cytoskeleton dynamics. During receptor-induced calcium signaling, PI(4,5)P2 is hydrolyzed by phospholipase C (PLC) to produce inositol triphosphate (IP3) that releases calcium from the endoplasmic reticulum (ER) to initiate calcium signaling. To sustain PI(4,5)P2-dependent functions during receptor-induced calcium signaling, PI synthesized in the ER is transferred to the PM to be converted to PI(4,5)P2, whereas phosphatidic acid (PA), a metabolic product of PI(4,5)P2, is transferred to the ER for PI synthesis. This homeostatic process maintaining the level of PI(4,5)P2 at the PM during receptor stimulation is known as the PI cycle.
Our research has revealed that
- Nir2 is a PI transfer protein that dynamically localizes to ER-PM junctions during calcium signaling
- E-Syt1 and Nir2 are important for replenishing PM PI(4,5)P2 after receptor-induced hydrolysis
- Nir2 mediates replenishment of PM PI(4,5)P2 with PI in the ER
- Nir2 and Nir3 mediate different levels of feedback regulation of PI(4,5)P2
- Intact F-actin network is required for Nir2-mediated PI(4,5)P2 homeostasis
- The level of PM PI(4,5)P2 is regulated by a novel RASSF4-ARF6-PIP5K pathway
We thank the funding sources for supporting our research.




Join Our Lab
Be part of the great impact we're having on science and medical care across the globe.Our lab is currently recruiting highly motivated graduate students and postdocs.