1. Mechanisms of protein misfolding and aggregation
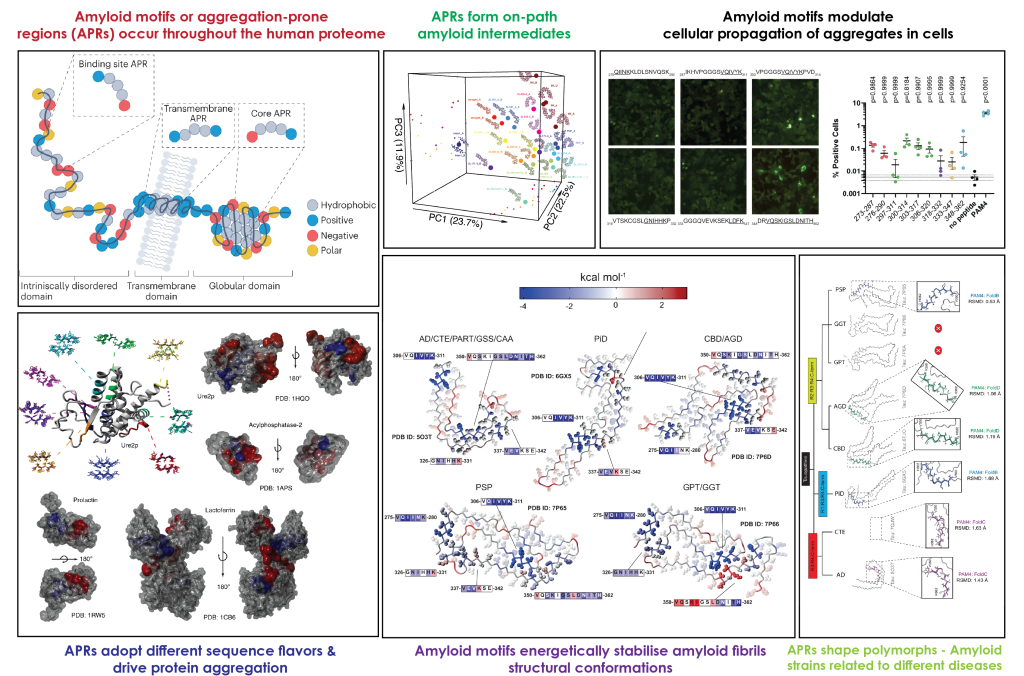
Investigating the molecular determinants behind protein aggregation and amyloid formation.
Amyloid motifs (AMs), also known as aggregation-prone regions (APRs), are short sequence segments (usually 5-15 residues in length) that promote the self-assembly of proteins into amyloid-like fibrillar aggregates.
These segments were initially linked to high hydrophobicity and beta-propensity, however, our work has expanded the amyloid sequence space revealing that APRs can also have high solubility and are frequently exposed on the surface of proteins.
Amyloid motifs act as "energetic staples" that stabilise the amyloid conformation. These local stretches also modulate amyloid polymorphism. Their intrinsic propensities promote proteins to adopt different amyloid structural architectures (amyloid strains / polymorphs) causing different types of disease.
Related publications
-
Mechanisms and pathology of protein misfolding and aggregation. Louros N, Schymkowitz J, Rousseau F. Nature Rev. Mol. Cell Biol. 24(12): 912-933. (2023)
-
Local structural preferences in shaping tau amyloid polymorphism. Louros N, et al. Nature Commun 15, 1028. (2024)
-
Thermodynamic analysis of amyloid fibril structures reveals a common framework for stability in amyloid polymorphs. #van der Kant R, #Louros N, et al. Structure 30(8): 1178-89 #equal contribution (2022)
-
Structure-based machine-guided mapping of amyloid sequence space reveals uncharted sequence clusters with higher solubilities. Louros N, et al. Nature Commun 11, 3314. (2020)
-
N-glycosylation as a eukaryotic protective mechanism against protein aggregation. Duran-Romaña R et al. Science Adv. 10, eadk8173. (2024)
2. Heterotypic Amyloid Interactions
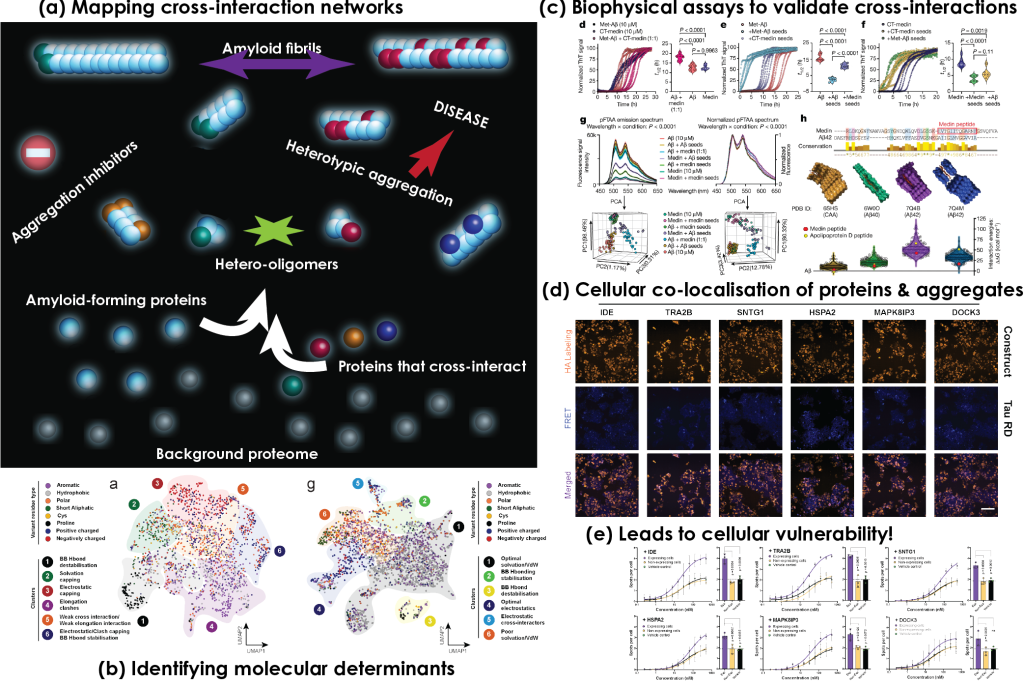
Heterotypic interactions modify polymorphism and toxicity of amyloid fibrils
-
Cross-interplay of amyloids with other biomolecules affects their structure, function and toxicity.
-
Heterotypic interactions, APRs and structurally frustrated regions mediate the formation of polymorphic amyloid structures.
- Interfaces promoting amyloid heterotypic interactions share different levels of specificity.
-
Cell-specific proteomic backgrounds can modulate amyloid toxicity and functionality through heterotypic-based assembly mechanisms.
Our previous work has shown that aggregates formed by proteins associated with major neurodegenerative diseases, such as tau and amyloid-beta peptide in Alzheimer's disease, participate in heterotypic interactions with important brain proteins that can alter their structural properties and modify their impact on disease. In our lab, we are interested in expanding our knowledge on how this cross-interplay of amyloids is an important contributor to neurodegenerative disorders.
Related publications
-
Medin promotes vascular amyloid-β aggregation in Alzheimer’s disease. Wagner J, Degenhardt K, Veit M, Louros N, et al. Nature 612, 123-131. (2022)
-
Mapping the sequence specificity of heterotypic amyloid interactions enables the identification of aggregation modifiers. Louros N, et al. Nature Commun 13, 1351 (2022)
-
Heterotypic amyloid interactions: clues to polymorphic bias and selective cellular vulnerability? Louros N, Schymkowitz J, Rousseau F. Curr. Opinion Struct. Biol. 72:176-186. (2021)
-
Heterotypic Aβ interactions facilitate amyloid assembly and modify amyloid structure. Konstantoulea K, Guerreiro P, Ramakers M, Louros N, et al. EMBO J, 41(2):e108591. (2021)
-
Heterotypic interactions in amyloid function and disease. Konstantoulea K, #Louros N, Rousseau F., Schymkowitz J. FEBS J 289: 2025-2046. #equal contribution (2021)
3. Development of therapeutic agents/inhibitors
Our lab has a long-standing interest towards the development of novel strategies and technologies that can be utilized to develop therapeutic agents against protein aggregation diseases. This includes, but also expands beyond major neurodegenerative disorders such as Alzheimer's and Parkinson's disease, to developing counteragents against biofilm formations or peptides targeting hard-to-drug proteins in various organisms.
Related publications
- Mapping the sequence specificity of heterotypic amyloid interactions enables the identification of aggregation modifiers. Louros N, et al. Nature Commun 13, 1351 (2022)
-
Enhanced therapeutic window for antimicrobial Pept-ins by investigating their structure-activity relationship. Wu G, Khodoparast L, Khodoparast L, De Vleeschouwer M, Louros N, Gallardo R, Yi P, Rousseau F, Schymkowitz J. PLOS ONE 18(3): e0283674. (2023)
-
Sequence-targeted Peptides Divert Functional Bacterial Amyloid Towards Destabilized Aggregates and Reduce Biofilm Formation. Sønderby T, Louros N, et al. J. Mol. Biol. 435 (11): 168039. (2023)
-
Exploiting the intrinsic misfolding propensity of the KRAS oncoprotein. Janssen K, et al. Proc. Nat. Acad. Sci. USA 120(9):e2214921120. (2023)
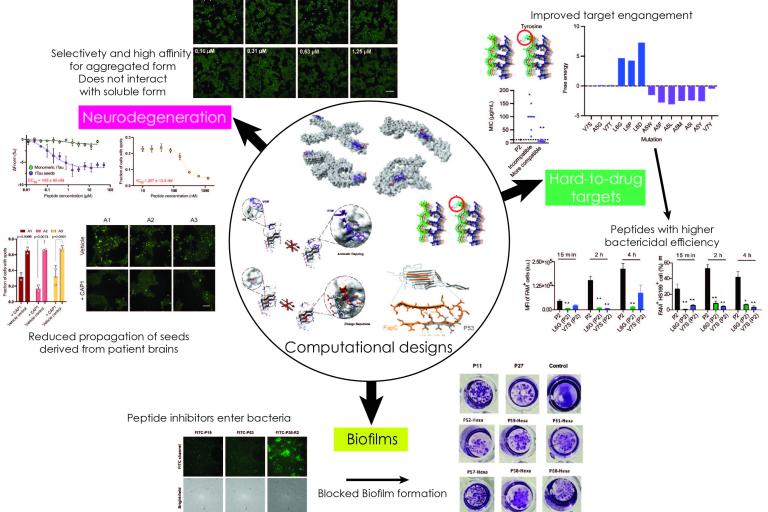 Therapeutic/biotechnological designs developed by our group - Starting from computational structure-based designs to patients/organisms
Therapeutic/biotechnological designs developed by our group - Starting from computational structure-based designs to patients/organisms 4. Functional amyloids! What, amyloids can be good?
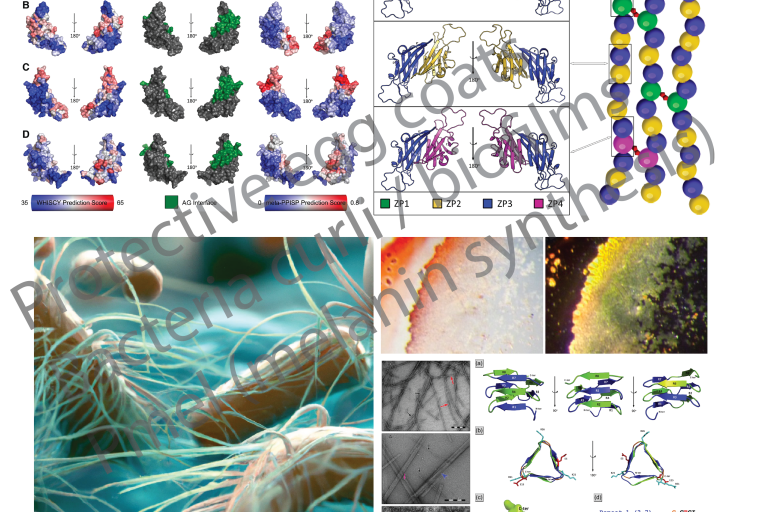 Mechanisms involving functional amyloid of interest to the Louros lab
Mechanisms involving functional amyloid of interest to the Louros lab Yes, amyloids are exploited by organisms!
Due to their association with disease, amyloids used to be considered as an aberration in biology. Yet, we now know that several organisms exploit the tremendous structural properties of amyloid to support important and complicated biological activities.
The Louros lab has a long-standing interest in studying the properties and impact of amyloids that support functional processes. This expands from protective coats of oocytes and storage or hormone-producing vesicles in the human body, to curli bacterial fibrils forming biofilms and proteins included in common food products!
Related publications
- Processing-induced changes in food proteins: amyloid formation during boiling of hen egg white. Monge-Morera M, Lambrecht M, Deleu L, Gallardo R, Louros N, De Vleeschouwer M, Rousseau F, Schymkowitz J, Delcour J. Biomacromolecules 21(6), 2218-28 (2020)
- Heating wheat gluten promotes the formation of amyloid-like fibrils. Monge-Morera M, Lambrecht M, Deleu L, Louros N, Rousseau F, Schymkowitz J, Delcour J. ACS Omega 6(3):1823 (2021)
-
Sequence-targeted Peptides Divert Functional Bacterial Amyloid Towards Destabilized Aggregates and Reduce Biofilm Formation. Sønderby T, Louros N, et al. J. Mol. Biol. 435 (11): 168039. (2023)
-
Hexapeptide tandem repeats dictate the formation of silkmoth chorion, a natural protective amyloid. #Tsiolaki P, #Louros N, Iconomidou V. J. Mol. Biol., 430: 3774-3783. (2018) #equal contribution
-
A common "aggregation-prone" interface possibly participates in the self-assembly of human zona pellucida proteins. Louros N, Chrysina ED, Baltatzis GE, Patsouris ES, Hamodrakas SJ, Iconomidou VA. FEBS Letters, 590: 619–630. (2016)
- Arabidopsis thaliana plant natriuretic peptide active domain forms amyloid-like fibrils in a pH-dependent manner. Nasi G, Aktipi F, Spatharas P, Louros N, et al. Plants 11(1):9 (2021)
5. Computational tools
 Computational tools developed by members of the Louros lab
Computational tools developed by members of the Louros lab Bioinformatics to the rescue!
Our lab members have a dedicated interest towards the development of software that can be used worldwide for studying protein folding, stability and aggregation