Germ Cell Differentiation
Post-transcriptional gene regulation has emerged as a critical control mechanism that orchestrates diverse cellular processes during germ cell differentiation. We have extensive preliminary data that demonstrate cytoplasmic Rbfox1 represses the translation of specific target mRNAs that contain (U)GCAUG elements within their 3’ untranslated regions during specific stages of germline-cyst differentiation.
Moving forward, we seek to characterize the detailed mechanisms by which cytoplasmic Rbfox1 regulates stage-specific mRNA translation programs during germ-cell cyst development. Our general strategy is to:
- Characterize how Rbfox1 regulates specific mRNAs—such as pumilio and sex-lethal—that govern early steps of germ cell development, and determine how ectopic expression of these targets contributes to Rbfox1-mutant phenotypes.
- Determine what mechanisms control the unique protein expression pattern of Rbfox1 during the intermediate stages of germline-cyst differentiation, before the onset of meiosis.
- Characterize how Rbfox1 molecularly regulates the translation of mRNA targets to achieve stage-specific translational repression.
We anticipate that these efforts will provide key insights into the translational control hierarchies that promote the development of germ cells across species.
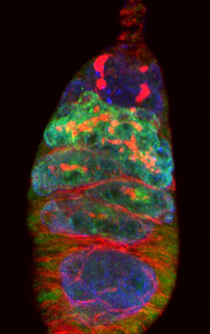 Germarium stained for Vasa (blue), Hts (red), and Rbfox1 (green).
Germarium stained for Vasa (blue), Hts (red), and Rbfox1 (green). Preservation of Genome Integrity
Perpetuation of a species requires that the genome be protected from endogenous and exogenous threats. Nowhere is this more critical than in the germ cells, the precursors of egg and sperm. Defects in the vigilance pathways lead to loss of genome integrity and subsequent miscarriage and/or deterioration of offspring vigor. Detailed studies of a newly identified, highly conserved genetic factor called germ cell nuclear acidic peptidase (GCNA), will provide novel insights into key determinants of genome integrity and fertility.
Our preliminary data show that loss of GCNA creates genomic instability in both Drosophila and C. elegans, indicating conservation of function. We are working on research that will provide some of the first insights into the shared mechanisms by which conserved GCNA family members promote genomic integrity and fertility across species.
To explore the mechanisms underlying gene functions in germ cell maintenance, we are studying:
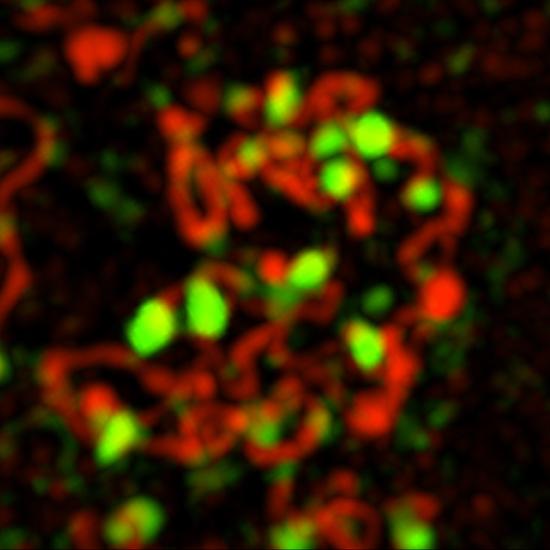 Germ cells are the only cells that undergo meiosis. Red marks the synaptonemal complex that indicates meiotic cells; green indicates double-stranded DNA breaks.
Germ cells are the only cells that undergo meiosis. Red marks the synaptonemal complex that indicates meiotic cells; green indicates double-stranded DNA breaks. - The classes of transposable elements that are activated in the mutant, their contribution to mutation accumulation, and the potential role of small RNA pathways.
- DNA repair pathways that require gcna function.
- The role of gcna in stability of repetitive elements.
- The contribution of gcna to cell cycle regulation using state-of-the-art imaging.
- The domain structure of GCNA
- GCNA's functional relevance to human fertility and germ cell tumors
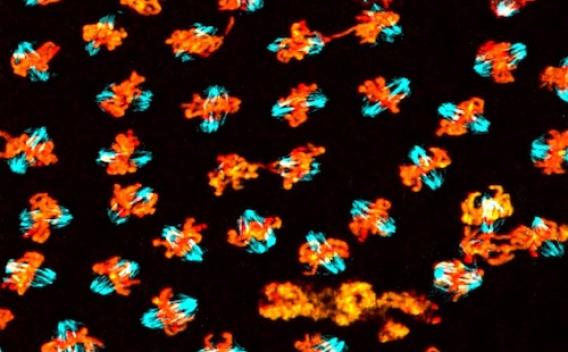 GCNA mutants show defects in chromosome segregation. Chromosomes are labeled orange and spindle fibers are labeled blue.
GCNA mutants show defects in chromosome segregation. Chromosomes are labeled orange and spindle fibers are labeled blue. Niche Function
Stem cells can replenish tissue throughout an organism's life. But they must also replenish themselves, maintaining a delicate balance that produces the correct proportion of differentiated daughter cells and new stem cells.
Specialized microenvironments called niches control this balance. Our long-term goal is to identify and characterize the factors that regulate niche function in vivo and to find out why stem cells and their niches become less active as an organism age. Mammalian tissues are unamenable to this work, so we seek to build upon previous work using the Drosophila ovary as a powerful model for stem-cell niches.
Our preliminary data suggest that niches carry out previously unrecognized functions that are likely to be important for stem-cell health and maintenance over time. Future studies include:
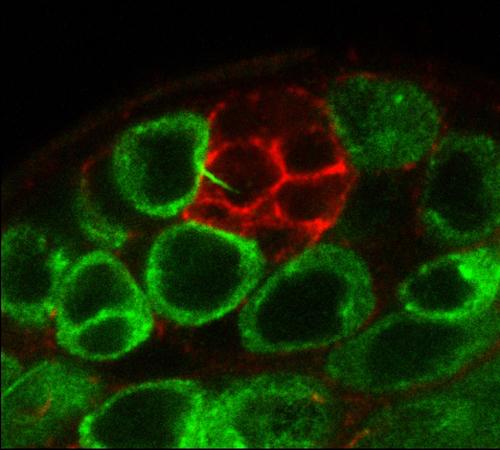 We have discovered novel microtubule-dependent extensions (MT-nanotubes) that foster BMP signaling within germline stem cells. At the upper left, a germ cell extends an MT-nanotube toward niche cells (red) in Drosophila testis.
We have discovered novel microtubule-dependent extensions (MT-nanotubes) that foster BMP signaling within germline stem cells. At the upper left, a germ cell extends an MT-nanotube toward niche cells (red) in Drosophila testis. - Assessing changes in gene expression within the Drosophila ovarian germline stem cell niche during aging.
- Testing whether niches help to protect stem cells from microbes introduced through natural routes or via injury.
- Probing the molecular mechanisms by which a superoxide dismutase helps to prolong proper niche function late in life.
This comprehensive analysis of in vivo niche function during aging will provide key insights into why stem cell activity declines with age and will reveal new molecular targets for the development of therapies to boost tissue maintenance and regeneration in aging organisms.
We have also explored how the size of the ovarian germline stem cell niche is limited. For example, we found that Lsd1 mutants produce a population of cells that resemble germline stem cells. Using a clonal analysis and cell-specific rescue experiments, we determined that Lsd1 does not act in the germline cells themselves, but rather in somatic cells outside of the normal stem cell niche. Further work showed that Lsd1 functions to keep these cells healthy and prevents them from acting as ectopic niches. These results suggest that cells immediately adjacent to the niche retain the capacity to express niche-specific signals. Such plasticity would allow niches to expand and contract in response to various environmental cues or injuries and potentially represents a common feature of in vivo stem cell niches across species.
This work has led us to begin to further characterize how germ cells interact with cells in their immediate vicinity, through direct physical contact and regulated signaling. Collaborating with Dr. Yukiko Yamashita at the University of Michigan, we have also discovered novel microtubule-dependent extensions (MT-nanotubes) that foster BMP signaling within germline stem cells of the Drosophila testis.