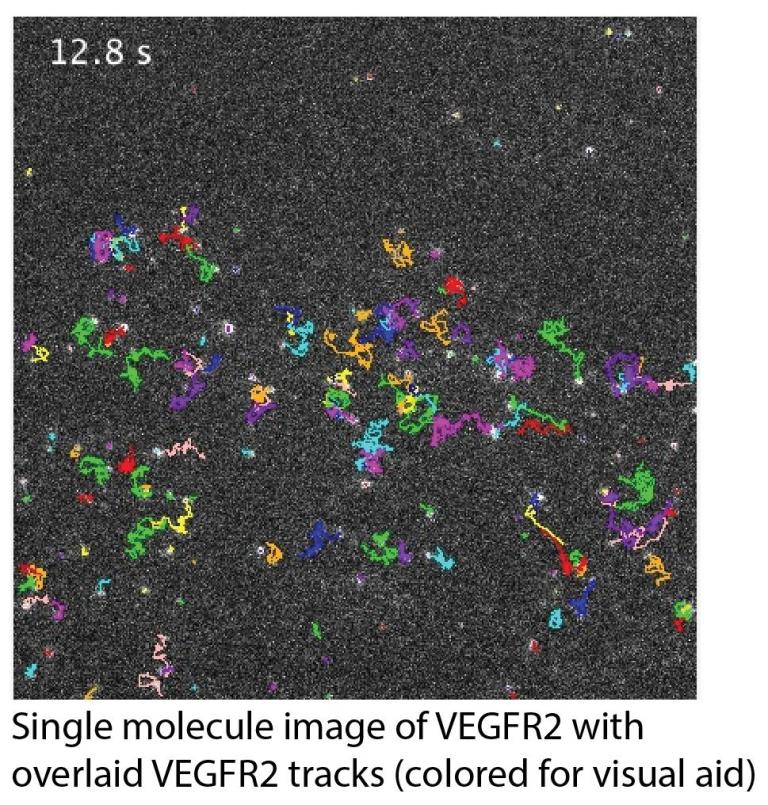
Spatiotemporal organization and signaling of VEGFR2 and its interaction partners
Vascular Endothelial Growth Factor (VEGF) Receptor 2 (VEGFR2), a receptor tyrosine kinase, is the main receptor for VEGF-A in endothelial cells, mediating its pro-angiogenic signaling (i.e. signaling to promote the growth of new blood vessels from the existing vasculature). There is evidence that VEGFR2 interacts with many receptors on the cell surface, including some that are anti-angiogenic (such as CD36). Yet many questions remain unanswered: How do these interactions influence VEGFR2 signaling? What is the dynamic organization of VEGFR2 and its interaction partners on the cell surface? Even regarding VEGR2 itself, is it monomeric or dimeric in its resting (i.e. unligated) state (a generally debated question in the RTK field)?
To address these questions, we are employing single-molecule imaging-based approaches, such as single-molecule tracking in live cells and super-resolution localization microscopy. We study single molecules to capture the full spectrum of molecular behaviors, including transient and rare events. We perform these studies in cells to link molecular behavior to its cellular/subcellular context and to capture the many factors that influence cell surface receptor organization.
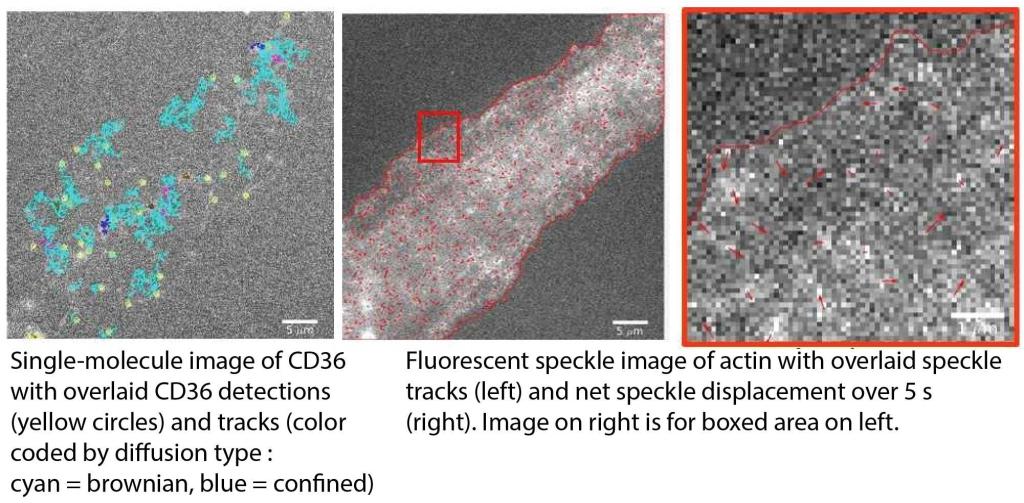
Actin cortex regulation of cell surface receptors
The actin cortex right underneath the plasma membrane highly influences the dynamics and organization of proteins and lipids in the plasma membrane. Initially, it was thought to primarily impede and compartmentalize the movement of membrane constituents. Later evidence suggested that it can also promote the movement and clustering of some receptors in the plasma membrane. Whichever the case, most studies derive the role of the actin cortex indirectly via perturbation experiments (including our previous work!). Yet, whole-cell actin perturbation might change the overall state of the cell and thus affect protein and lipid behavior in the plasma membrane for reasons unrelated to the actin cortex.
To avoid these problems and directly derive the influence of the actin cortex on membrane receptor organization, we are developing multi-color, high-resolution imaging approaches that allow us to simultaneously monitor the actin cortex (using quantitative fluorescent speckle microscopy) and membrane proteins (using single-molecule imaging).
This project has got us into the world of “big data”! Our challenge now is to develop the proper analytical tools (machine learning!) to make sense of all the data that we have been collecting.
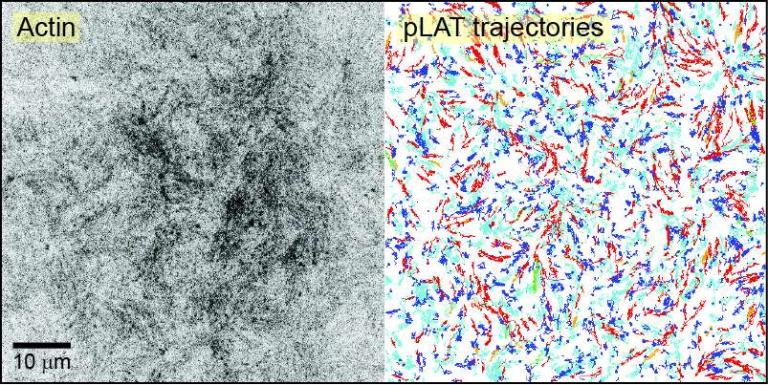
Movement and compositional regulation of phase-separated LAT clusters in T cell membranes
Phase separation of proteins (and nucleic acids) is an emerging mechanism for the organization of molecules into dynamic, micron-scale foci without a surrounding membrane. These condensates are involved in many processes, including transmembrane signal transduction.
A prime example of such condensates is the plasma membrane-associated clusters that form during T cell activation, composed of the adapter protein LAT and various binding partners. Interestingly, these biomolecular condensates interact with actin, where on the one hand the condensates regulate actin filament assembly, and on the other hand, actin filaments help move the condensates across the immunological synapse, necessary for proper T cell signaling.
We are interested in understanding the interplay between actin and the movement and composition of these biomolecular condensates. To achieve this, we are collaborating with the Rosen Lab here at UTSW, with whom we are developing quantitative microscopy and associated analytical techniques to study these condensates, both in cells and in vitro.