Vascular Image-Guided Optimization of Response (VIGOR) to Therapy in Kidney Cancer
We will develop the application of a promising novel vascular disrupting agent (VDA) in combination with leading therapies to enhance treatment of kidney cancer. Renal cell carcinoma (RCC) is usually characterized by inactivation of the von Hippel Lindau (vHL) tumor suppressor protein, promoting accumulation of Hypoxia Inducible Factor (HIF) and consequent development of extensive vasculature. The endothelium of normal blood vessels is largely quiescent, but the invasive neovasculature of tumors is immature, lacks pericyte support, and exhibits increased permeability providing a selective target for cancer therapy. The therapeutic goal of VDAs is to cause rapid widespread disruption of established tumor vasculature leading to regional ischemia, induction of hypoxia and tumor necrosis.
We have identified OXi8007 as a new potent, water-soluble VDA prodrug generating protracted vascular disruption, dose dependent tumor growth delay and no apparent systemic toxicity. However, VDA monotherapy generally results in re-growth at the tumor periphery and OXi8007 will likely be most effective in augmenting current lead therapies based on complementary modes of action. We will investigate a small-molecule HIF-2 antagonist, PT2977 developed by Peloton Therapeutics, which represents a new class of chemotherapeutic in early clinical trials. Meanwhile, cabozantinib, the small- molecule kinase inhibitor that targets the c-MET receptor, AXL, and VEGFR-2 was recently approved as a first line treatment option for patients with metastatic RCC. We also recognize the emerging success of immunotherapy and anticipate that OXi8007-induced necrosis will enhance antigen presentation promoting response.
Our overarching hypothesis is that combining these therapeutic approaches will achieve robust long term control of RCC. Investigations will benefit from the resources of the UT Southwestern Kidney Cancer SPORE, which has developed a number of new patient derived tumor lines exhibiting differential sensitivity to HIF-2 antagonists. Effective therapy combination will likely depend on timing of administration of the respective agents and non- invasive imaging will reveal the spatial and temporal pharmacodynamics of tumor response. Bioluminescence imaging (BLI) will effectively interrogate luciferase-transfected RENCA cells in immunocompetent mice. In addition, recently available multispectral optoacoustic tomography (MSOT) non-invasively reveals vascular extent and regional oxygenation without the need for exogenous reporter molecules or cell transfection. Complementary cell-based studies are designed to further explore OXi8007 mechanism of action. Effectively combining targeted therapies should enhance treatment and ultimately survivorship of kidney cancer patients. The goal of these investigations is to demonstrate effective combination therapy as a foundation for investigations in large animals and translation to the clinic.
References
Wanniarachchi HI, Schuetze R, Deng Y, Hamal KB, Pavlich CI, Tankoano PEO, Tamminga C, Hammers H, Kapur P, Bueno LMA, Rayas R, Wang T, Liu L*, Trawick ML, Pinney KG, Mason RP*. Evaluating Therapeutic Efficacy of the Vascular Disrupting Agent OXi8007 Against Kidney Cancer in Mice. Cancers (Basel). 2025 Feb 24;17(5):771. doi: 10.3390/cancers17050771. PMID: 40075618; PMCID: PMC11898701.
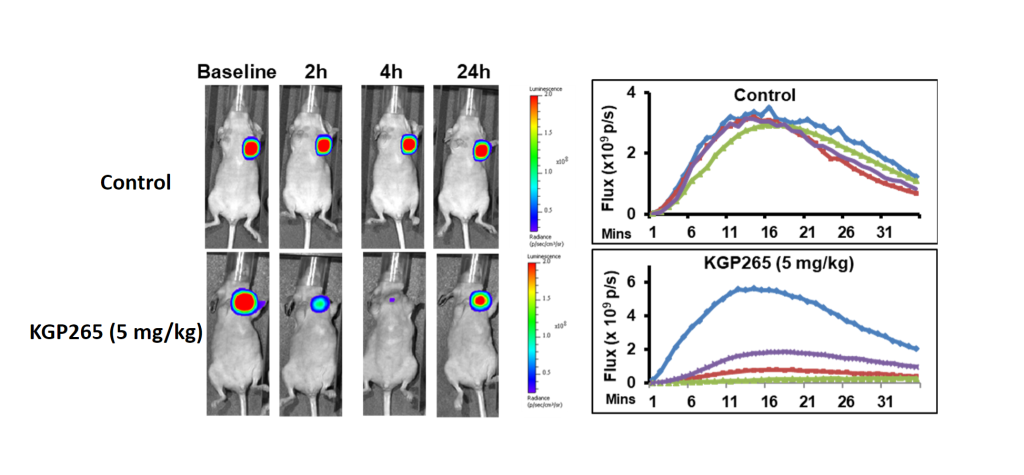
Guo Y, Wang H, Gerberich JL, Odutola SO, Charlton-Sevcik AK, Li M, Tanpure RP, Tidmore JK, Trawick ML, Pinney KG, Mason RP, Liu L, Imaging-Guided Evaluation of the Novel Small-Molecule Benzosuberene Tubulin-Binding Agent KGP265 as a Potential Therapeutic Agent for Cancer Treatment. Cancers (Basel) 2021 Sep 13 19
Liu L, O'Kelly D, Schuetze R, Carlson G, Zhou H, Trawick ML, Pinney KG, Mason RP, Non-Invasive Evaluation of Acute Effects of Tubulin Binding Agents: A Review of Imaging Vascular Disruption in Tumors., Molecules 2021 Apr 26 9
Liu L, Schuetze R, Gerberich JL, Lopez R, Odutola SO, Tanpure RP, Charlton-Sevcik AK, Tidmore JK, Taylor EA-S, Kapur P, Hammers H, Trawick ML, Pinney KG, Mason RP. Demonstrating Tumor Vascular Disrupting Activity of the Small-Molecule Dihydronaphthalene Tubulin-Binding Agent OXi6196 as a Potential Therapeutic for Cancer Treatment. Cancers. 2022; 14(17):4208. https://doi.org/10.3390/cancers14174208
Hashini I. Wanniarachchi, Lorena M. A. Bueno, Ricardo Rayas, Khagendra B. Hamal, Cyprian I. Pavlich, Mary Lynn Trawick, Kevin G. Pinney, Li Liu, Ralph P. Mason, "Acute vasculature disruption in orthotopic kidney tumors based on vascular disrupting agent (OXi8007) examined by multispectral optoacoustic tomography (MSOT)," Proc. SPIE 12842, Photons Plus Ultrasound: Imaging and Sensing 2024.