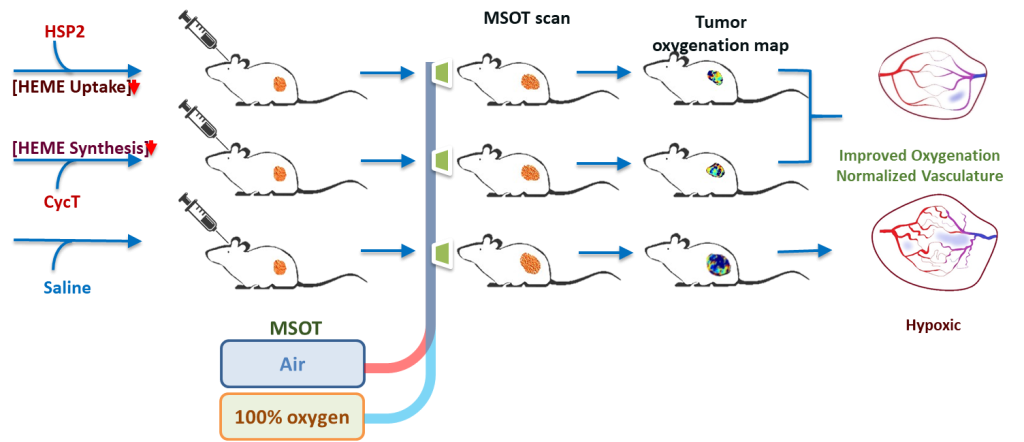
Dr. Liu’s research focuses on the development of reporter genes and the evaluation of reporter gene applications in preclinical cellular and molecular imaging, particularly in small animal models of breast, prostate, lung, and brain cancers, including subcutaneous, orthotopic, and lung colonization tumor models. Using molecular optical imaging techniques, Dr. Liu has conducted extensive evaluations with transfected cell lines and tumor models to investigate the efficacy of emerging technologies, such as biodegradable photoluminescent polymers and functional polymers for siRNA delivery. Most recently, she has applied multispectral optoacoustic tomography (MSOT) to noninvasively examine tumor vasculature in real time, demonstrating that two experimental drugs can normalize aberrant blood vessels, improve oxygenation, and modulate the tumor microenvironment in non small cell lung cancer, thereby suppressing tumor growth and metastasis.
Dr. Liu’s expertise lies in the development and evaluation of novel therapeutic strategies using dynamic bioluminescent, fluorescent, and MSOT imaging, with a particular emphasis on vascular disrupting agents and heme targeting drugs.
For more information: