Breast cancer cells interact closely with the bone microenvironment, a process that promotes tumor growth, drug resistance, and osteolytic bone destruction, ultimately worsening patient outcomes. Bone marrow stromal cells play a central role in this process by secreting factors that support cancer cell homing, survival, dormancy, and activation of bone resorbing osteoclasts.
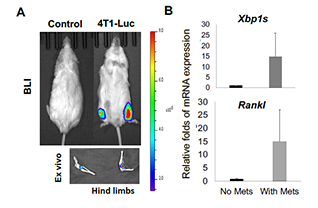
The bone tumor microenvironment is highly inflammatory, hypoxic, and nutrient deprived, conditions that activate endoplasmic reticulum stress signaling. X box binding protein 1 spliced (XBP1s), a key effector of this pathway, regulates protein folding and secretion required for stromal cell function. Our previous work identified XBP1s as a stromal intrinsic driver of tumor growth and bone destruction in multiple myeloma. Notably, XBP1s has also been implicated in drug resistance and relapse in aggressive breast cancer.
Building on these findings, this project investigates the role of the stromal IRE1α XBP1s pathway in supporting breast cancer growth and bone destruction. Understanding this mechanism may reveal new strategies to disrupt tumor stromal interactions and improve treatment of breast cancer bone metastasis.