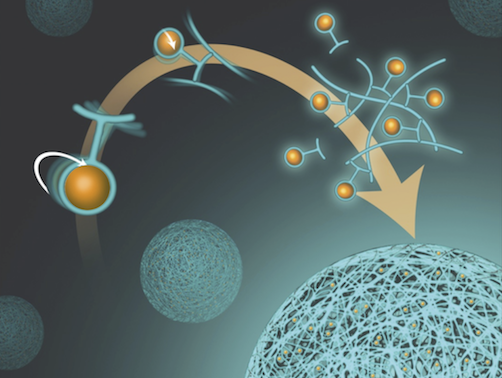
The Lux lab is part of the Translational Research in Ultrasound Theranostics (TRUST) Program.
Our research interests lie in the development of novel platforms to diagnose and treat disease in vivo noninvasively.
Our group focuses on three main research topics:
- Ultrasound-guided immunotherapy of cancer
- Targeted and activatable ultrasound contrast agents
- Bioresponsive nanomaterials for in vivo detection of disease and therapy
Our long-term objective is to develop clinically translatable imaging agents and immunotherapeutics.

Current Projects
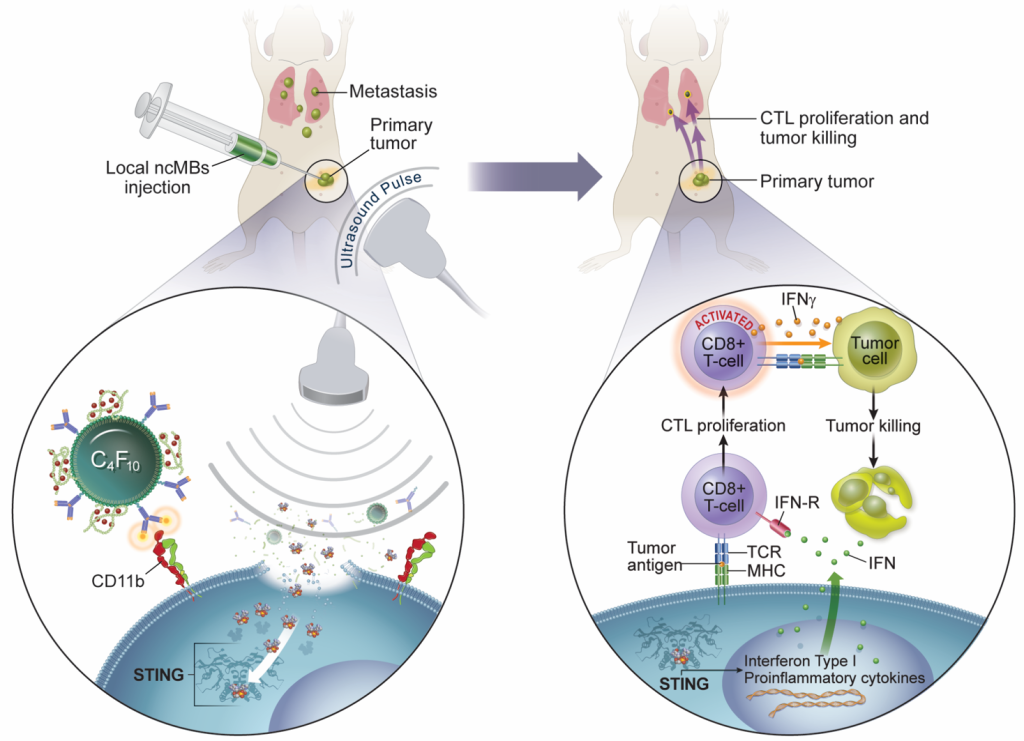
Microbubble-assisted Ultrasound-guided Immunotherapy of Cancer (MUSIC)
There is an urgent and unmet need to develop more effective immunotherapy strategies for cancer patients. In order to generate optimal antitumor immunity, both the innate and adaptive branches of the body’s immune systems need to be engaged. This understanding has led to the development of new immunotherapies that target regulators of innate immune systems, including agonists that stimulate the cytosolic DNA sensor, cyclic GMP-AMP Synthase-Stimulator of Interferon Genes pathway (cGAS-STING) pathway. However, STING agonists such as cyclic GMP-AMP (cGAMP) cannot penetrate the cell membrane easily, which is required to activate downstream antitumor immune responses, and thus severely limits their clinical potential. I have developed a microbubble-assisted ultrasound (US)-guided immunotherapy for cancer (MUSIC) that reengineers clinically approved microbubbles to load and deliver cGAMP into APCs under US stimulation with spatiotemporal control with the use of a preclinical sonoporator. We showed that MUSIC produced robust STING activation and type I interferon responses in APCs and more efficiently primed antigen-specific CD4+ and CD8+ T cells in vitro. In collaboration with Wen Jiang, MD, PhD demonstrated that these immune stimulatory effects directly translated into antitumor effects against syngeneic orthotopic (EO771) and metastatic (4T1) murine breast cancer models. Both models showed dramatic anti-tumor responses following local treatment of the primary tumor, with 60% of animals becoming tumor-free after 50 days in the case of the EO771 tumors and a significant decrease of the systemic disease burden including lung metastases in the case of the 4T1 breast cancer.
Li X, Khorsandi S, Wang Y, Santelli J, Huntoon K, Nguyen N, Yang M, Lee D, Lu Y, Gao R, Kim BYS, de Gracia Lux C, Mattrey RF, Jiang W*, Lux J*. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat Nanotechnol. 2022 Aug;17(8):891-899. doi: 10.1038/s41565-022-01134-z. Epub 2022 May 30. PMID: 35637356; PMCID: PMC9378430.
Development of activatable ultrasound contrast agents
Patients with acute deep vein thrombosis (DVT) are difficult to distinguish from patients with chronic DVT by imaging alone. The distinction is important, as patient with acute DVT require anti-coagulation to stop clotting, which can potentially lead to fatal pulmonary embolisms. As thrombin activity is indicative of active clotting, I developed a thrombin-specific microbubble (MB) ultrasound contrast agent to help recognize patients that would benefit from anti-coagulation. The thrombin-specific activation of this contrast agent was demonstrated with an in vitro flow phantom. Recently, I reported the first example of hydrogel-cloaked MBs, as well as activatable MBs that can easily be engineered to be responsive to various biomarkers of disease. In this work pH was used as a biomarker of choice to validate this proof of concept, and I plan to use this platform to develop targeted and thrombin-sensitive MBs for the detection of acute DVT.
I also helped in the formulation and characterization of low boiling point perfluorocarbon nanodroplets as phase-change contrast agents. Those agents have a great potential for imaging and therapy as their small size allows extravasation and they can be converted to echogenic microbubbles when exposed to ultrasound. Recently, Finally I recently collaborated with Dr. de Gracia Lux to develop a new strategy to inflate microbubbles using low boiling point perfluorocarbon nanodroplets.
Burns MWN, Mattrey RF, Lux J. Microbubbles Cloaked with Hydrogels as Activatable Ultrasound Contrast Agents. ACS Appl Mater Interfaces. 2020 Nov 25;12(47):52298-52306. doi: 10.1021/acsami.0c12043. Epub 2020 Nov 10. PMID: 33170637.
Brambila CJ, Lux J, Mattrey RF, Boyd D, Borden MA, de Gracia Lux C. Bubble Inflation Using Phase-Change Perfluorocarbon Nanodroplets as a Strategy for Enhanced Ultrasound Imaging and Therapy. Langmuir. 2020 Mar 24;36(11):2954-2965. PubMed PMID: 32090572.
Lux J, Vezeridis AM, Hoyt K, Adams SR, Armstrong AM, Sirsi SR, Mattrey RF. Thrombin-Activatable Microbubbles as Potential Ultrasound Contrast Agents for the Detection of Acute Thrombosis. ACS Appl Mater Interfaces. 2017 Nov 1;9(43):37587-37596. PubMed PMID: 28994575; PubMed Central PMCID: PMC5691601.
de Gracia Lux C, Vezeridis AM, Lux J, Armstrong AM, Sirsi SR, Hoyt K, Mattrey R. Novel method for the formation of monodisperse superheated perfluorocarbon nanodroplets as activatable ultrasound contrast agents. RSC advances. 2017 October 16; 7:48561-48568.
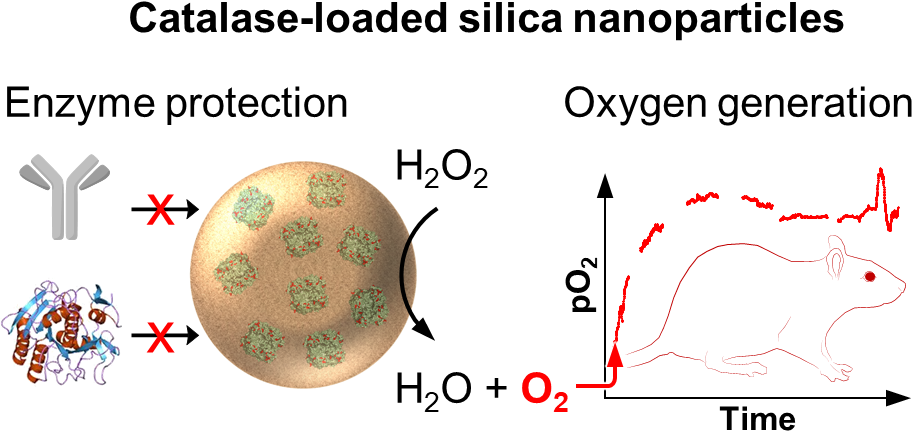
Design and Synthesis of Activatable Probes and Nanoprobes
The ability to visualize biochemical changes related to disease has a high clinical impact, as it could lead to earlier, more accurate diagnosis than conventional structural imaging. During my postdoc, I successfully designed and synthesized new fluorescent dyes with improved two-photon brightness for RNA and DNA labeling. As an assistant research scientist, I designed new dual-triggered optical nanoprobes capable of simultaneously detecting acidosis and oxidative stress associated with inflammatory microenvironments. To complete this project, I successfully coordinated the efforts of a multidisciplinary team of materials scientists, chemical engineers and clinicians to obtain promising in vivo imaging results in inflammatory diseases with target-to-background ratios reach 22 as early as 3 h post-injection. As a PI in the TRUST program, I developed catalase-containing nanoparticles to image oxidative stress. The first generation of those agents, that were comprised of a hydrogel core and a silica shell were used to successfully determine infected from non-infected abscess fluid collections in humans. Recently, we improved the formulation of these catalase-loaded nanoparticles to yield particles with high catalase activity and maximal protection against proteases for in vivo application. The ultimate goal of this project is to develop oxygen nanogenerators to decrease tumor hypoxia and sensitize them against radiation therapy.
Heble AY, Santelli J, Armstrong AM, Mattrey RF, Lux J. Catalase-Loaded Silica Nanoparticles Formulated via Direct Surface Modification as Potential Oxygen Generators for Hypoxia Relief. ACS Appl Mater Interfaces. 2021 Feb 10;13(5):5945-5954. doi: 10.1021/acsami.0c19633. Epub 2021 Jan 26. PMID: 33497181.
Malone CD, Fetzer DT, Lux J, Mattrey RF. Catalase-Containing Silica Particles as Ultrasound-Based Hydrogen Peroxide Sensors to Determine Infected From Noninfected Fluid Collections in Humans. AJR Am J Roentgenol. 2019 Mar 12;PubMed PMID: 30860893.
Viger ML, Collet G, Lux J, Nguyen Huu VA, Guma M, Foucault-Collet A, Olejniczak J, Joshi-Barr S, Firestein GS, Almutairi A. Distinct ON/OFF fluorescence signals from dual-responsive activatable nanoprobes allows detection of inflammation with improved contrast. Biomaterials. 2017 Jul;133:119-131. PubMed PMID: 28433935; PubMed Central PMCID: PMC5704950.
Lux J, Peña EJ, Bolze F, Heinlein M, Nicoud JF. Malachite green derivatives for two-photon RNA detection. Chembiochem. 2012 May 29;13(8):1206-13. PubMed PMID: 22549874.
Join Our Lab
A postdoctoral training position is available in the laboratory of Dr. Jacques Lux, in the in the Translational Research for Ultrasound Theranostics (TRUST) program within the Department of Radiology at UT Southwestern Medical Center to study, develop and translate enzyme-based nanoparticles for in vivo detection of disease and therapy.Graduate Student
If you are interested in joining the lab as a graduate student, please apply to the UT Southwestern graduate school.
Postdoctoral position
If you are interested in a position as a postdoctoral researcher in the TRUST research program, please email a detailed CV to Email.