About Prostate Cancer
In 2017, there will be approximately 161,360 new cases of Prostate Cancer (PCa) diagnosed and 26,730 deaths in the United States, according to the American Cancer Society. Mortality from PCa can be attributed to its metastasis to bone. For metastatic PCa, androgen deprivation therapy (ADT) is the initial treatment of choice and results in disease regression. However, ADT is not curative: the cancer invariably relapses and progresses to castrate-resistant PCa (CRPC). CRPC is often treated with secondary hormone therapies that block the production of androgens or their ability to interact with androgen receptor (AR) and subsequent AR activation. Despite recent FDA approval of chemotherapeutic and immunomodulatory agents, CRPC is incurable and most patients with CRPC will die.
About Breast Cancer
According to 2017 estimates from the American Cancer Society, there will be approximately 252,710 new cases of invasive breast cancer diagnosed and 40,610 deaths among women in the United States. Like Prostate Cancer, the most common site of metastasis in breast cancer is the bone. The treatment options for breast cancer are largely dependent on the specific type a patient has. Estrogen receptor (ER) or progesterone receptor (PR) positive patients will likely begin a course of treatments to block estrogen or progesterone production or target the receptor that senses these hormones. Human epidermal growth factor type 2 receptor (HER2/neu) positive patients are likely to be treated with drugs that target the HER2/neu protein, like pertuzumab or trastuzumab. Currently, the treatment options for patients negative for all three receptors, otherwise known as triple-negative breast cancer, are limited; however, these patients may receive targeted therapies to block DNA damage repair, like PARP inhibitors. Despite the growing number of various types of targeted therapies for breast cancer, the 5-year relative survival rate for metastatic breast cancer remains around 22%.
Hormone receptor-dependent signaling
Our primary focus is to molecularly characterize signaling pathways in prostate and breast cancers. We believe that these studies will help uncover exploitable vulnerabilities within the cancers.
We know that CRPC is characterized by active androgen receptor (AR) signaling, which dictates resistance to therapies. AR is noted to be predominantly nuclear in CRPC and binds to cognate DNA response elements to mediate a robust gene expression programme. Up to 5 percent of genes in CRPC may be regulated by AR. AR forms a productive transcription complex on chromatin in conjunction with several coregulatory proteins (coactivators and corepressors) and collaborating transcription factors.
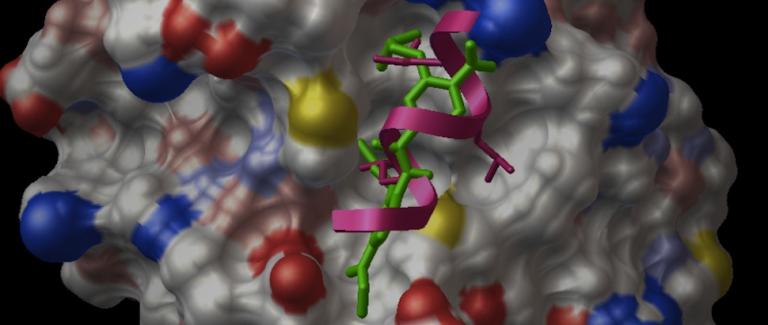
The interaction between AR and these coactivators is critical for AR signaling and we endeavor to understand the molecular basis of these interactions and their functional import. We characterized the interaction between AR and a coactivator PELP1 and showed that this interaction could couple AR signaling with the activation of other steroid receptors. This novel mechanism of steroid receptor cross-talk has been validated in other systems and may represent an important resistance mechanism in prostate cancer. Current work focuses on potential targeting of this steroid receptor cross-talk in prostate cancer. We are also evaluating the importance of the interaction between AR and other critical coactivators in prostate cancer, including FoxA1, src, hsp27 and hsp90 in AR signaling in CRPC.
ERα is a driver of hormone-dependent breast cancer development and is found in over 70% of breast cancers. Estradiol and other agonists activate ER, which is then able to directly function as a transcription factor or indirectly regulate cell cycle, survival, growth factor signaling, and other pathways. Treatment of ER-positive tumors with anti-estrogens/aromatase inhibitors is often effective; however, resistance to such treatments is common via the maintenance of ER signaling through oncogenic coregulators. Over a third of ER coregulators, including SRC3, SRC2, and PELP1, are overexpressed in breast cancers. This upregulation allows ER signaling to be sustained in the presence of antiestrogens, which are essentially converted to agonists to promote tumorigenesis, resistance, and metastasis. The nuclear receptor box motif in the ER is crucial for interactions with coregulators. In order to inhibit interactions between the ER and coregulators and block ER signaling, we have developed a number of small organic molecules to mimic this motif.
Blocking signaling in hormone dependent cancers
Our studies of protein-protein interactions critical for AR signaling, has led to fundamental breakthroughs. With Jung-Mo Ahn, Ph.D., of UT Dallas, we have developed a transformative class of drugs targeting specific protein-protein interactions central to AR function in prostate cancer. These drugs known as peptidomimetics are orally bioavailable, potent, non-toxic small molecules that specifically target the interface between AR and specific cofactors. One such molecule, the D2 peptidomimetic (named D2 or Drug 2) was designed to block the interaction between AR and cofactors containing a LXXLL motif (L=leucine, X= any amino acid) and was the subject of our recent manuscript in Nature communications. The peptidomimetics can target specific sequences in specific structures, making them potent molecules for disrupting specific interactions or genres of interactions between proteins. Ongoing work in the laboratory is focused on both advancing these drugs to clinic and to develop additional agents against other critical protein-protein interactions.
Deciphering mechanisms of therapy resistance
Resistance to hormonal therapies is common in prostate and breast cancers, and is mediated by multiple mechanisms. Resistance can be mediated by amplification or mutations of the hormonal receptor, use of secondary nuclear receptors, alterations in the coregulator profile. The Raj Laboratory is particularly interested in understanding mechanisms of enzalutamide resistance in prostate cancer and tamoxifen resistance in breast cancer.
Development of new models
A major impediment to the clinical translation of research findings is the lack of preclinical models that can accurately predict the clinical efficacy of new drugs and, therefore, enable the selection of agents that are most suitable for clinical trials. An approach that we developed with Wayne Tilley is the ex vivo culture of primary human tissues, which retains the native tissue architecture, hormone responsiveness, and cell-to-cell signaling of the tumor microenvironment in a dynamic and manipulable state. Ex vivo culture systems recapitulate the structural complexity and heterogeneity of human prostate cancers in a laboratory setting, making them an important adjunct to current cell-line-based and animal-based models. When incorporated into preclinical studies, ex vivo cultured tissues enable robust quantitative evaluation of clinically relevant end points representing drug efficacy, investigation of therapy resistance, and biomarker discovery. By providing new clinically relevant insights into prostate carcinogenesis, it is likely that ex vivo culture will enhance drug development programmes and improve the translational ‘hit rate’ for prostate cancer research. We have the largest experience in the world with the ex vivo technique and have published extensively with this technique. Our current research is focused on developing second and third generation culture techniques for reliable drug response evaluation.
Decoding radiation resistance
While radiation therapy is commonly used for treating patients with prostate cancer, radiation resistance can significantly limit long-term control of the disease. A molecular understanding of why radiation therapy fails is critical to developing better therapies in these patients. We have identified an actionable mechanism-based strategy to improve treatment outcomes in localized prostate cancer. These findings provide a mechanistic rationale for therapeutic targeting of DNA-PK in the context of combined hormonal therapy and radiotherapy as a strategy to radiosensitize clinically localized prostate cancer. Our current research focuses on incorporating ex vivo culture of prostate tumors in understanding how prostate cancers response to radiation and the development of radiosensitizers, with a goal of improving the efficiency of translating preclinical research to clinical practice.
Biomarkers
Our lab is committed to the “bench to bedside” translation of our research endeavors, leading to systemic evaluation of mechanisms of therapy resistance. We have assembled a panel of resistant prostate cancer cell lines that do not respond to the current FDA approved drugs or those in development. Using a systemic bioinformatics approach, we have identified a panel of genes that may have predictive value for both responsiveness to primary radiation therapy and advanced anti-androgen therapy. This ongoing and exciting work we hope will lead to the delineation of a predictive and prognostic biomarker panel of molecular drivers in prostate cancer.