
The Wert Laboratory studies the photoreceptor neurons of the retina, the primary light-sensing cells that are responsible for excitation to the inner neuronal cell layers of the retina for visual processing of optical images by the brain. Although mutations in more than 280 genes have now been linked to retinal degenerative disease – characterized by the death of the photoreceptor neurons – there are currently no available treatment options to prolong vision and halt cell death for the majority of these diseases and the mechanism by which the photoreceptors degenerate is not well understood. Determining the downstream cellular responses that impact photoreceptor development, maturation, and maintenance can provide insight needed to develop a therapeutic approach to treat patients with retinal dystrophies.
We model these complex human diseases using both genetically engineered mice (for in vivo studies of neuronal cell development and health over time) as well as human cell lines – including pluripotent stem cells – for in vitro studies of 3D retinal organoid development and maturation of the retinal neurons. The long-term goal of the Wert Laboratory is to discover and understand the mechanisms underlying retinal neurodegenerative disease, and to provide novel therapeutics for these complex disorders.
The Role of Modifying Factors Such as Sex and Metabolism on Retinal Degenerative Disease
The Wert Laboratory has a strong interest in determining the mechanisms underlying photoreceptor degeneration in inherited retinal dystrophies. We have a particular interest in modifying factors that alter the rate of disease progression. At this time, the Wert laboratory is investigating how sex and sex hormone signaling affect the retina and retinal degenerative disease. We also examine how dietary changes and metabolism on the rate of retinal degeneration and health of the photoreceptors. Identification of the impact for various modifying factors, such as sex and diet, on the retina in both a healthy and disease state will better inform critical pathways underlying photoreceptor degeneration, proper study design, as well as patient care.
How Gene Deregulation Affects Photoreceptor Development and Maintenance
The Wert Laboratory is actively working to uncover the gaps in our understanding of the molecular mechanisms that cause photoreceptor degeneration. To investigate this, we study how genes associated with photoreceptor degeneration affect their normal development, maturation and maintenance over time. We use both genetic animal models of disease, as well as human pluripotent stem cells to study human retinal development and disease in a dish. These studies pave the way to further understand how disease mutations can affect photoreceptor formation and health.
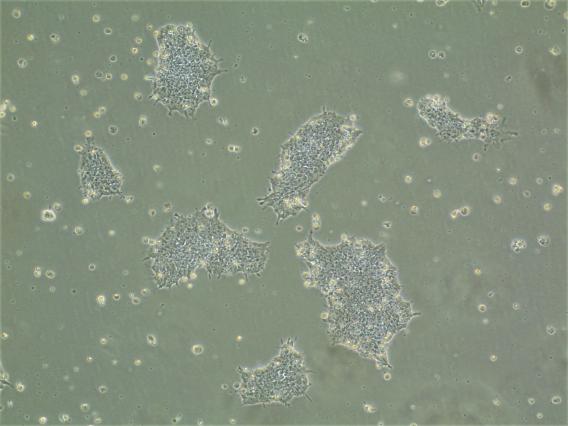 Human embryonic stem cells
Human embryonic stem cells 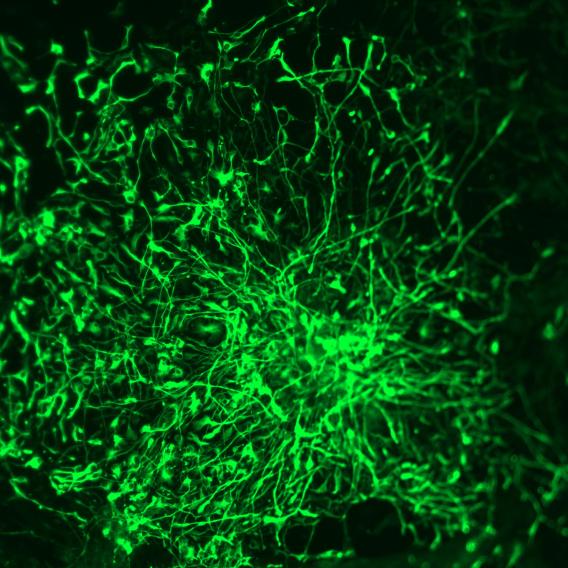 Human stem cells differentiated into neural precursor cells
Human stem cells differentiated into neural precursor cells 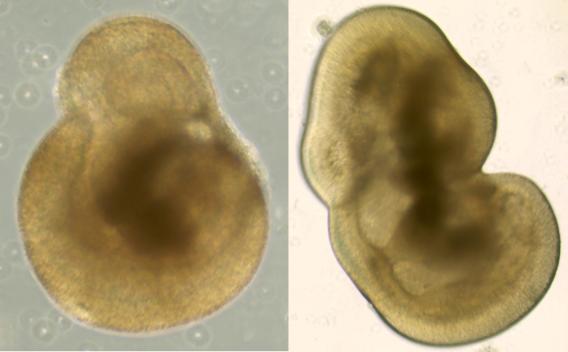 Human stem cells differentiated into 3D retinal organoids
Human stem cells differentiated into 3D retinal organoids Gene Therapy in the Eye
Dr. Wert has previously developed an approach to deliver viral vectors, drugs, and/or cells into the subretinal and intravitreal space of the perinatal and adult mouse eye that can be performed with minimal equipment by a single investigator. The Wert Laboratory has the ability to use this approach to test gene therapy efficacy in preclinical mouse models with retinal degenerative diseases, as well as to validate Genome-wide Association Studies (GWAS) risk alleles for their role in ocular pathology.
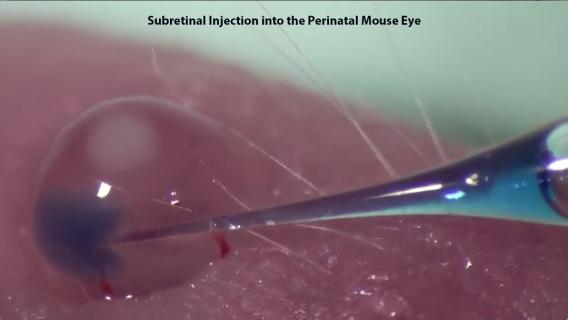 Subretinal and Intravitreal Injections
Subretinal and Intravitreal Injections