The host and the intestinal microbiota form a complex, interacting ecosystem so that nutrients are exchanged and protection is provided for each to maintain health and homeostasis. New bacteria introduced into the ecosystem must overcome both physical and metabolic hurdles to establish a niche in this ecosystem.
Campylobacter jejuni is a commensal organism in the intestinal tracts of many animals in the wild and agriculture, including avian, bovine, and porcine species. However, infection of the human intestinal tract often progresses to diarrheal disease. Although the outcomes of infection are different, C. jejuni colonizes the lower intestinal tract of both the human and avian intestinal tracts. To colonize these areas, C. jejuni must identify favorable niches that support growth by recognizing specific nutrients and other components. Proper signaling networks in the bacterium must be able to sense specific cues and then alter gene transcription accordingly to promote colonization.
We discovered that C. jejuni can sense microbiota-generated metabolites in the human and avian intestines to spatially discern different regions of the intestines. We found that C. jejuni senses physiologically-relevant concentrations of lactate, acetate, and butyrate, and then changes transcription of virulence and/or colonization factors required for infection of humans to promote diarrheal disease or colonization of avian species to promote commensalism. Acetate and butyrate produced by the microbiota are abundantly present in the lower intestinal regions where C. jejuni predominantly colonizes in hosts. As such, C. jejuni senses these metabolites and augments the expression of colonization genes. In contrast, lactate is present in greater concentrations in the upper intestinal tract than in the lower intestinal regions; C. jejuni responds to lactate by reducing the transcription of colonization genes. These data allowed us to develop a model whereby C. jejuni monitors intestinal concentrations of microbiota-generated metabolites such as butyrate, acetate, and lactate as spatial markers to identify prime intestinal niches for colonization such as the lower intestinal tract, and then alter gene expression to promote colonization.
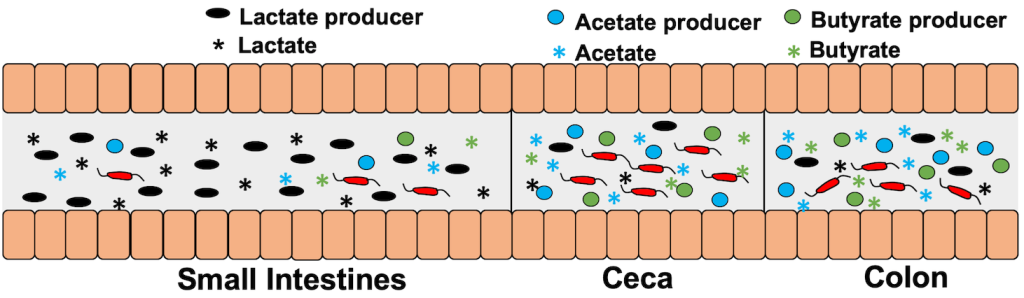 Spatial distribution of certain members of the microbiota and microbiota-generated metabolites in the intestinal tract of hosts colonized by C. jejuni
Spatial distribution of certain members of the microbiota and microbiota-generated metabolites in the intestinal tract of hosts colonized by C. jejuni We are exploring how C. jejuni can sense these microbiota-generated metabolites and identifying systems responsible for specifically sensing these metabolites. We have initially identified the BumSR two-component signal transduction system (TCS) as one such system that indirectly senses and responds to butyrate. We have explored the signal transduction mechanism of the BumSR system and discovered its mechanism is unusual. Instead of functioning as a histidine kinase to phosphorylate its cognate BumR response regulator in a signal-dependent manner, the BumS sensor appears to strictly possess phosphatase activity for phospho-BumR. We are currently exploring this unique signal transduction mechanism and identifying other specific systems and mechanisms for sensing lactate, acetate, and other microbiota-generated metabolites.
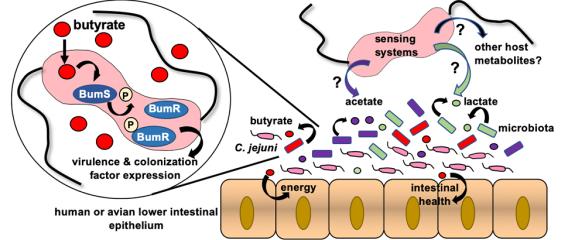 A proposed mechanism to sense intestinal butyrate by the C. jejuni BumSR two-component signal transduction system
A proposed mechanism to sense intestinal butyrate by the C. jejuni BumSR two-component signal transduction system Relevant Publications
Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract
Hendrixson, D. R., and DiRita, V. J. Mol Microbiol (2004) 53:471-84
Analysis of the activity and regulon of the two-component regulatory system composed by Cjj81176_1484 and Cjj81176_1483 of Campylobacter jejuni
Luethy, P. M., Huynh, S., Parker, C. T., and Hendrixson, D. R. J. Bacteriol (2015) 197:1592-605
Microbiota-derived short-chain fatty acids modulate the expression of Campylobacter jejuni determinants required for commensalism and virulence
Luethy, P. M., Huynh, S., Ribardo, D. A., Winter, S. E., Parker, C. T., and Hendrixson, D. R. mBio (2017) 8:e00407-17
Campylobacter jejuni BumSR directs the response to butyrate via sensor phosphatase activity to impact transcription and colonization
Goodman, K. N., Powers, M. J., Crofts, A. A., Trent, M. S., and Hendrixson, D. R. Proc Natl Acad Sci (2020) 117:11715-11726