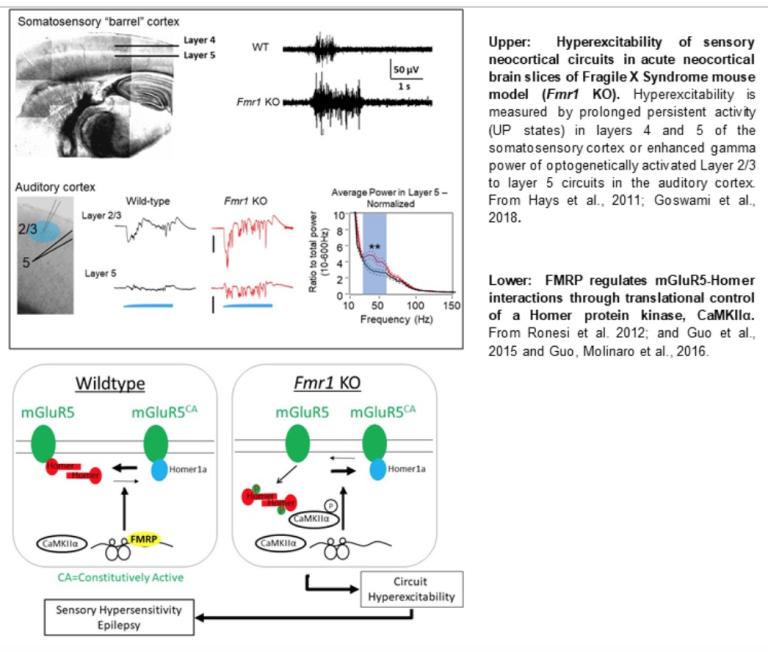
In addition to our work on mechanisms of FMRP function at synapses, we have also contributed to the understanding of circuit dysfunction in Fragile X Syndrome, revealing mechanisms of FMRP function in the brain, but also to identify therapeutic targets to correct and restore circuit function in diseases such as FXS and related autistic disorders A common symptom in FXS and autism is sensory hypersensitivity and evidence suggests this is due to hyperexcitability of sensory circuits. In collaboration with my colleague, Dr. Jay Gibson, we discovered alterations in synaptic function and connectivity of sensory neocortical circuits in the FXS mouse model, Fmr1 KO which lead to hyperexcitability of circuits in the somatosensory and auditory neocortex (Gibson et al., 2008, Hays et al., 2011, Ronesi et al., 2012; Goswami et al., 2019). We work within an NIH Center for Collaborative Research in Fragile X (U54) where we collaborate with mouse and human researchers to understand and correct sensory circuit hyperexcitability in FXS mouse models and develop neurophysiological biomarkers of sensory circuit dysfunction that translate from mouse to humans with FXS to predict and monitor treatment outcomes (Goswami et al., 2019).
Hyperactivity of the Gq-coupled metabotropic glutamate receptor 5 (mGluR5) contributes to the hyperexcitable circuits and seizures in the Fmr1 KO mouse. We discovered a molecular mechanism of mGluR5 hyperactivity in FXS that occurs as a result of disrupted interactions with its interacting and scaffolding protein Homer (Ronesi and Huber, 2008, Ronesi et al., 2012; Guo et al., 2016). We also discovered a molecular pathway by which FMRP regulates mGluR5-Homer interactions through translational suppression of a Homer kinase, CaMKIIα (Guo et al., 2015). MGluR5-driven hyperexcitability of circuits is also implicated in other genetic causes of autism and related diseases, such as obsessive-compulsive disorder (Ade et al., 2016). Current projects seek to understand the molecular, synaptic, and cellular basis by which mGluR5 controls circuit function and how this is regulated by autism-linked genes, such as FMRP. We also seek to understand how synaptic scaffolding proteins, such as Homer, control mGluR5 function at synapses, and how this is regulated in the normal brain by experience and learning. This work is funded by an NIH, NICHD U54 grant.