New mechanisms of immune checkpoint regulation
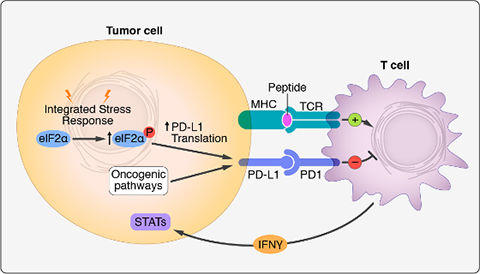
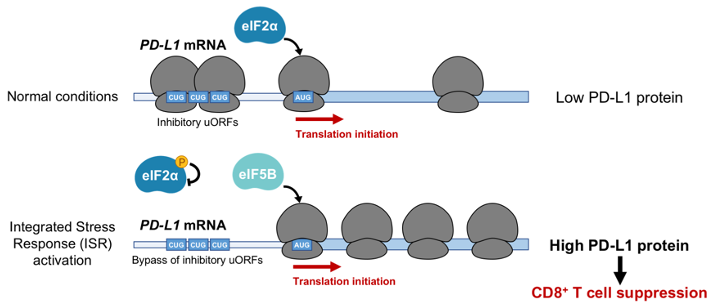
Cancer cells express high levels of PD-L1, a ligand of the PD-1 receptor on T cells, allowing tumors to suppress T cell activity. Clinical trials utilizing monoclonal antibodies that disrupt the PD-1/PD-L1 immune checkpoint have yielded remarkable results, with PD-1 immunotherapy approved as a first-line therapy for human lung cancer patients. Despite significant progress in targeting this pathway, the mechanisms through which PD-L1 is upregulated in non-small cell lung cancer (NSCLC) remain incompletely understood. To address this question, we applied CRISPR-based screening to identify regulators of PD-L1 in human lung cancer cells. Surprisingly, this work revealed potent induction of PD-L1 levels upon disruption of the heme biosynthesis pathway. We discovered that heme depletion activates the integrated stress response (ISR), inducing bypass of inhibitory upstream open reading frames (uORFs) in the human PD-L1 5' UTR (Suresh et al., Nature Cancer, 2020). This culminated in enhanced PD-L1 translation and suppression of anti-tumor immunity. We further demonstrated that ISR-dependent translation of PD-L1 requires the alternative translation initiation factor eIF5B. Remarkably, eIF5B overexpression is sufficient to induce PD-L1 in the absence of ISR. Because eIF5B is commonly overexpressed in human lung cancer and is associated with poor prognosis, our results illuminate mechanisms of immune checkpoint activation and identify new targets for therapeutic intervention.
Currently, we are continuing to investigate mechanisms of PD-L1 regulation, and elucidate the complete translational program orchestrated by eIF5B in lung cancer.