Investigating the role of PCDH7 in lung cancer pathogenesis
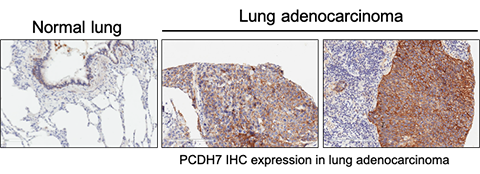
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-associated deaths worldwide. Given the exciting potential of targeting membrane proteins in human malignancies using therapeutic antibodies, we sought to identify cell-surface receptors that act as drivers of lung tumorigenesis. We recently reported that PROTOCADHERIN 7 (PCDH7), a transmembrane receptor and member of the Cadherin superfamily, is frequently overexpressed in NSCLC tumors, and high expression of PCDH7 is associated with poor clinical outcome (Zhou et al., Cancer Research, 2017). We showed that PCDH7 overexpression synergizes with KRAS and EGFR (the two most commonly mutated oncogenes in NSCLC) to induce MAPK signaling and tumorigenesis. Moreover, PCDH7 depletion suppressed ERK activation, sensitized cells to MEK inhibitors, and reduced tumor growth. Our mechanistic studies revealed that PCDH7 potentiates ERK signaling by facilitating interaction of Protein Phosphatase 2A (PP2A) with its potent inhibitor, the SET oncoprotein. These findings establish a previously uncharacterized oncogenic role for PCDH7 in lung tumorigenesis and provide proof-of-concept support for the development of novel therapies that target PCDH7 at the cell surface of NSCLC cells.
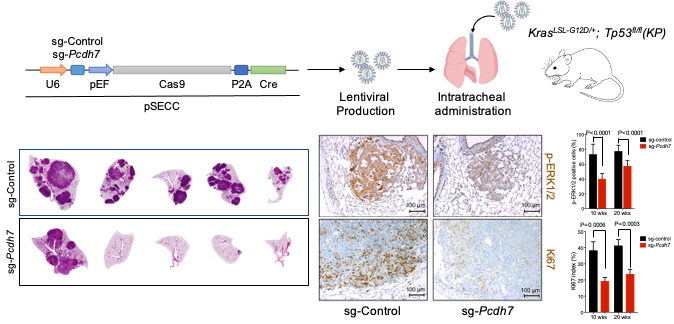
We next sought to rigorously examine whether PCDH7 has oncogenic activity in lung cancer using genetically engineered mouse models. Utilizing a transgenic model developed in our laboratory, we demonstrated that gain-of-function of PCDH7 accelerates KrasG12D-driven lung tumorigenesis and potentiates MAPK pathway activation (Zhou et al., Molecular Cancer Research 2019). Importantly, these data provided the first in vivo evidence that modulation of PCDH7 impacts KrasG12D-driven lung tumorigenesis. We also employed somatic genome editing with virally delivered CRISPR in the KrasLSL-G12D; Tp53fl/fl (KP) model to validate PCDH7 as a new therapeutic target in lung cancer. Inactivation of PCDH7 in KP mice significantly reduced lung tumor development, prolonged survival, and diminished phospho-activation of ERK1/2. Taken together, this study established PCDH7 as an oncogenic driver of tumor initiation and progression, providing further support for efforts to target this cell-surface protein in lung adenocarcinoma.
Currently, we are investigating the mechanisms through which PCDH7 promotes oncogenesis and the role of PCDH7 in EGFR-driven tumorigenesis. We are also developing therapeutic antibodies that block PCDH7 function.
Currently, we are investigating the mechanisms through which PCDH7 promotes oncogenesis and the role of PCDH7 in EGFR-driven tumorigenesis. We are also developing therapeutic antibodies that block PCDH7 function.