Research
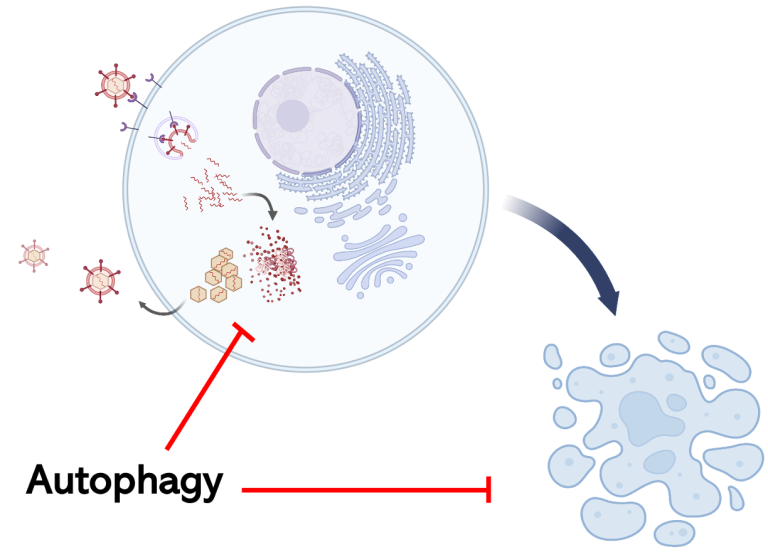
The relationship between autophagy, virus infection and neuroinflammation is complex but central to host survival from virus infection of the central nervous system. Autophagy is a cellular process involved in the capture and degradation of cellular components including damaged organelles and protein aggregates. It plays a crucial role in maintaining cellular homeostasis particularly in response to various stress conditions such as viral infections. Autophagy can directly engulf and degrade viruses or viral components, a process called virophagy. In so doing, autophagy disrupts viral replication, participates in antigen presentation, regulates proinflammatory signaling pathways, and promotes cell survival. For neurons, which are terminally differentiated, autophagy plays a critical role in mitigating virus-induced cell death allowing for the survival of the host. Viruses can manipulate the autophagic process as a mechanism of immune evasion. Dysregulated autophagy can lead to the development of excessive or prolonged neuroinflammation and accumulation of damaged organelles such as mitochondria and aberrant protein aggregates that disrupt neuronal function.
One major outstanding question that the Thinwa lab is interested in is how does autophagy discriminately recognize viruses or viral components?
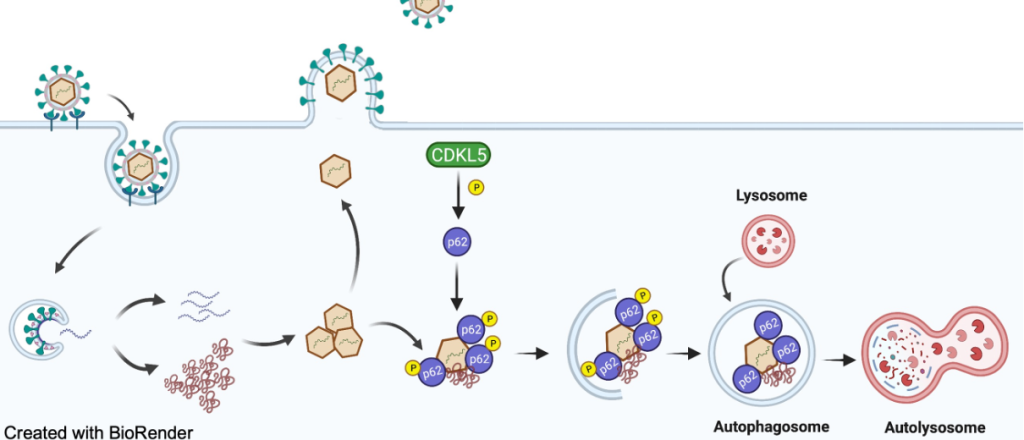
We identified CDKL5 (Cyclin-Dependent Kinase-Like 5), a protein kinase essential in brain development and neuronal function, as a novel regulator of virophagy. We found mechanistically CDKL5 functions to turn on autophagy by regulating the ability of autophagy receptors to bind onto viral components. But CDKL5 is just the tip of the iceberg. We have taken an in vivo transcriptomic and proteomic approach to identify numerous host proteins and pathways that intersect with autophagy and CDKL5. These targets and pathways will reveal mechanisms of autophagy activation and how autophagy interfaces with antiviral immune response pathways.
Mapping out the process of autophagy activation during viral infection could help us find ways to circumvent steps that are inhibited by viruses and discover how to turn on autophagy specifically during virus infection as a potential antiviral therapeutic target.
The Thinwa lab studies neurotropic viruses, host defense pathways, autophagy and brain development