Basal suppression of Shh signaling by Gpr161
The primary cilium is critical for Shh signaling in dorso-ventral neural tube patterning (Goetz and Anderson, 2010). Shh signaling culminates in processing of the bifunctional Gli transcription factors to generate Gli activators or repressors in a cilium-dependent manner. Addition of Shh results in removal of Patched (Ptch1), the Shh receptor from cilia, and accumulation of Smoothened (Smo), a frizzled family GPCR in the compartment.Smo determines activation of the pathway by promoting generation of Gli2 activator.
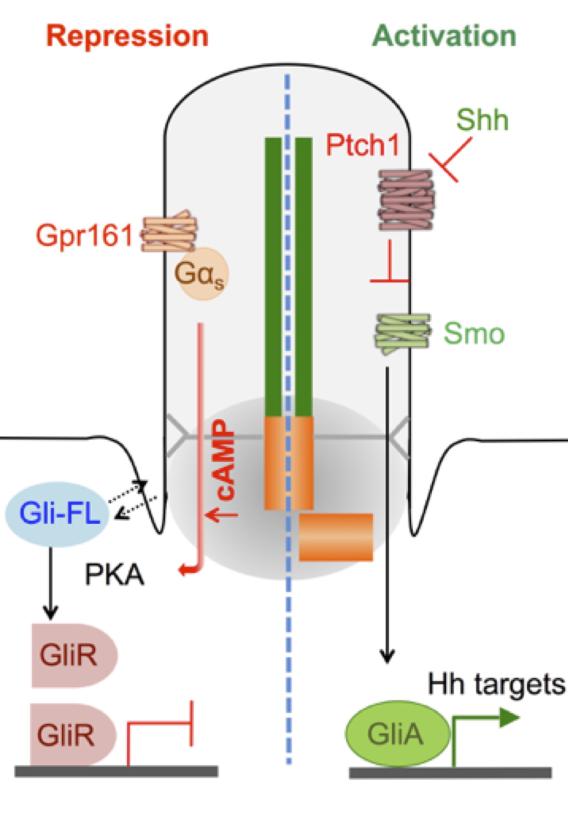 Model of Gpr161 in the Shh pathway. Gli3FL, Gli3 full-length; Gli3R, Gli3 repressor; GliA, Gli activator
Model of Gpr161 in the Shh pathway. Gli3FL, Gli3 full-length; Gli3R, Gli3 repressor; GliA, Gli activator In addition to Gli activator, Gli repressors (mainly Gli3R) are generated in a cilium-, protein kinase A- (PKA), and suppressor of fused (Sufu)-dependent manner upon proteolytic processing (Mukhopadhyay and Rohatgi, 2014), referred to as the basal repression machinery of Shh signaling. However, the pathways determining cAMP-PKA signaling in the cillium are not well understood and are critical toward addressing the role of negative regulation of the pathway.
Gpr161 is an orphan GPCR highly expressed in the neural tube during embryonic development. Our recent work defines Gpr161 as a negative regulator of the Shh pathway by functioning in the basal suppression machinery (Mukhopadhyay et al., 2013). Complete loss of Gpr161 in embryos results in embryonic lethality by E10.5, and ventralization throughout the rostro-caudal extent of the neural tube. The mutant embryos also exhibit extensive craniofacial abnormalities, with open fore/mid brain regions, and caudal neural tube defects (Mukhopadhyay et al., 2013). Gpr161 is trafficked to the primary cilia (Mukhopadhyay et al., 2013). Gpr161 knockout embryos lack Gli3 repressor, and Gpr161 constitutively activates cAMP formation, suggesting that Gpr161 activates cyclic AMP-dependent PKA signaling in promoting Gli repressor formation in a primary cilia-dependent manner (Mukhopadhyay et al., 2013).
Limb and skeletal development
The role of basal suppression of Shh pathway and its interaction with Indian hedgehog (Ihh) signaling during limb/skeletal morphogenesis is not well understood. We discovered that forelimb buds are not formed in Gpr161 knockout mice embryos despite establishment of prospective limb fields. Limb-specific deletion of Gpr161 resulted in prematurely expanded Shh signaling and ectopic Shh-dependent patterning defects to cause polysyndactyly. In addition, endochondral bone formation in forearms, including formation of both trabecular bone and bone collar was prevented.
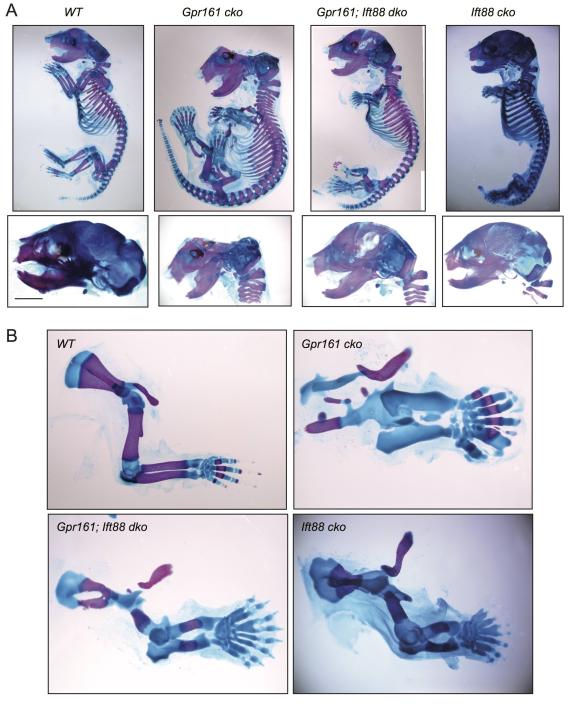 Limb and skeletal phenotypes in Gpr161 conditional knockouts
Limb and skeletal phenotypes in Gpr161 conditional knockouts Endochondral bone formation defects resulted from accumulation of proliferating round/periarticular-like chondrocytes, lack of differentiation into columnar chondrocytes, and corresponding absence of Ihh signaling. Gpr161 deficiency in craniofacial mesenchyme also prevented intramembranous bone formation in calvarium. Defects in limb patterning, endochondral and intramembranous skeletal morphogenesis were suppressed in the absence of cilia. Overall, Gpr161 promotes forelimb formation, regulates limb patterning, prevents periarticular chondrocyte proliferation, and drives osteoblastogenesis in intramembranous bones in a cilium-dependent manner (Hwang et al., 2018).
Cerebellum development and medulloblastoma pathogenesis.
Shh determines cerebellar granule cell (GC) progenitor proliferation and medulloblastoma pathogenesis. However, pathways regulating GC progenitors during embryogenesis before Shh production by Purkinje neurons and their roles in tumorigenesis remain unclear. By deleting Gpr161 in mouse neural stem cells or GC progenitors, we establish Gpr161 as a tumor suppressor in Shh-subtype medulloblastoma. Irrespective of Shh production in cerebellum, Gpr161 deletion increased downstream activity of the Shh pathway by restricting Gli3-mediated repression, causing more extensive generation and proliferation of GC progenitors.
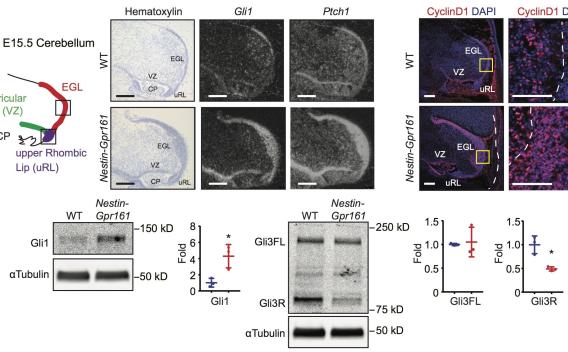 Increased Shh signaling in Gpr161 conditional mutant cerebellum in the absence of Shh.
Increased Shh signaling in Gpr161 conditional mutant cerebellum in the absence of Shh. Moreover, earlier deletion of Gpr161 during embryogenesis increased tumor incidence and severity. GC overproduction during embryogenesis from Gpr161 deletion was cilia-dependent, unlike normal development. Low GPR161 expression correlated with poor survival of SHH-subtype medulloblastoma patients. Together, Gpr161 restricts GC production by preventing premature and Shh-dependent pathway activity, highlighting importance of basal pathway suppression in tumorigenesis (Shimada et al., 2018).
Forebrain and ventricular development
Inverse gradients of transcriptional repressors antagonize the transcriptional effector response to morphogens. However, the role of such inverse regulation might not manifest solely from lack of repressors. Sonic hedgehog (Shh) patterns the forebrain by being expressed ventrally; however, absence of antagonizing Gli3 repressor paradoxically cause insufficient pathway activation. Interestingly, lack of the primary cilia-localized G-protein-coupled receptor, Gpr161 increases Shh signaling in the mouse neural tube from coordinated lack of Gli3 repressor and Smoothened-independent activation. Here, by deleting Gpr161 in mouse neuroepithelial cells and radial glia at early mid-gestation we detected derepression of Shh signaling throughout forebrain, allowing determination of the pathophysiological consequences. Accumulation of cerebrospinal fluid (hydrocephalus) was apparent by birth, although usual causative defects in multiciliated ependymal cells or aqueduct were not seen. Rather, the ventricular surface was expanded (ventriculomegaly) during embryogenesis from radial glial overproliferation. Cortical phenotypes included polymicrogyria in the medial cingulate cortex, increased proliferation of intermediate progenitors and basal radial glia, and altered neocortical cytoarchitectonic structure with increased upper layer and decreased deep layer neurons. Finally, periventricular nodular heterotopia resulted from disrupted neuronal migration, while the radial glial scaffold was unaffected. Overall, suppression of Shh pathway during early mid-gestation prevents ventricular overgrowth, and regulates cortical gyration and neocortical/periventricular cytoarchitecture (Shimada et al., 2019).
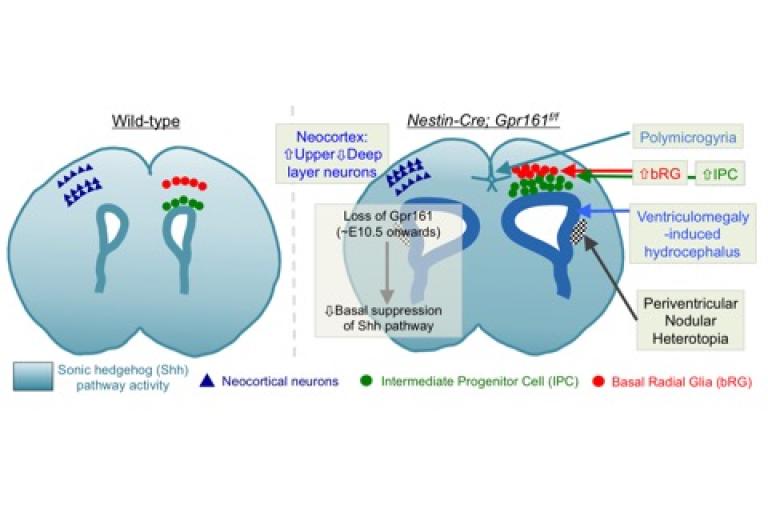 Derepression of Shh signaling upon Gpr161 deletion unravels forebrain and ventricular abnormalities.
Derepression of Shh signaling upon Gpr161 deletion unravels forebrain and ventricular abnormalities. References
Goetz, S.C., and Anderson, K.V. (2010). The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11, 331-344.
Hwang, S.H., White, K.A., Somatilaka, B.N., Shelton, J.M., Richardson, J.A., and Mukhopadhyay, S. (2018). The G protein-coupled receptor Gpr161 regulates forelimb formation, limb patterning and skeletal morphogenesis in a primary cilium-dependent manner. Development 145.
Mukhopadhyay, S., and Rohatgi, R. (2014). G-protein-coupled receptors, Hedgehog signaling and primary cilia. Semin Cell Dev Biol 33, 63-72.
Mukhopadhyay, S., Wen, X., Ratti, N., Loktev, A., Rangell, L., Scales, S.J., and Jackson, P.K. (2013). The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell 152, 210-223.
Shimada, I.S., Hwang, S.H., Somatilaka, B.N., Wang, X., Skowron, P., Kim, J., Kim, M., Shelton, J.M., Rajaram, V., Xuan, Z., et al. (2018). Basal Suppression of the Sonic Hedgehog Pathway by the G-Protein-Coupled Receptor Gpr161 Restricts Medulloblastoma Pathogenesis. Cell Rep 22, 1169-1184.